It strongly encourages investigator-initiated research across the spectrum of its mission. The NIH issues hundreds of funding opportunity announcements (FOAs) in the form of programme announcements (PAs) and requests for applications (RFAs) to stimulate research in particular areas of science. Some PAs, called ‘Parent Announcements’, span the breadth of the NIH mission in order to ensure we have a way to capture ‘unsolicited’ applications that do not fall within the scope of targeted announcements. The majority of NIH applications are submitted in response to PAs.
Types of grant programmes: NIH’s investigator-initiated research funding falls under two categories: (i) Research Grants (R series) and (ii) Programme Project/Centre Grants (P series). In addition, the NIH supports funding in other categories: (iii) Training and Fellowships (T & F series), (iv) Resource Grants, (v) Career Development Awards and (vi) Trans-NIH Programmes. The NIH uses activity codes (e.g. R01, R43, P01, etc.) to differentiate the wide variety of research-related programmes.
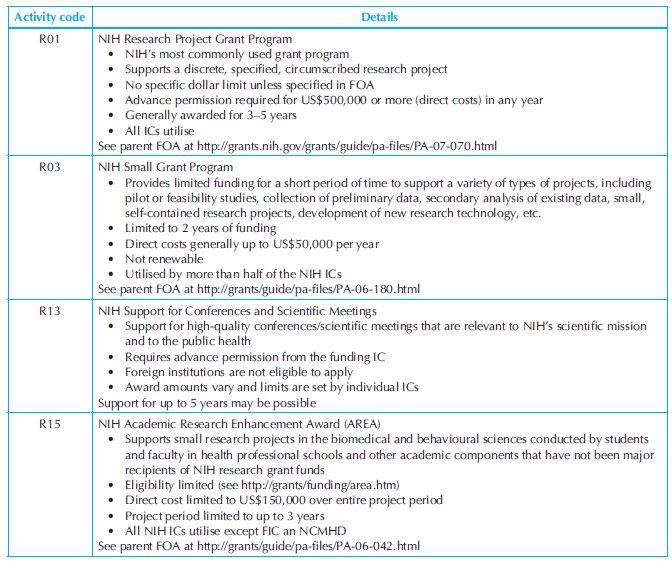
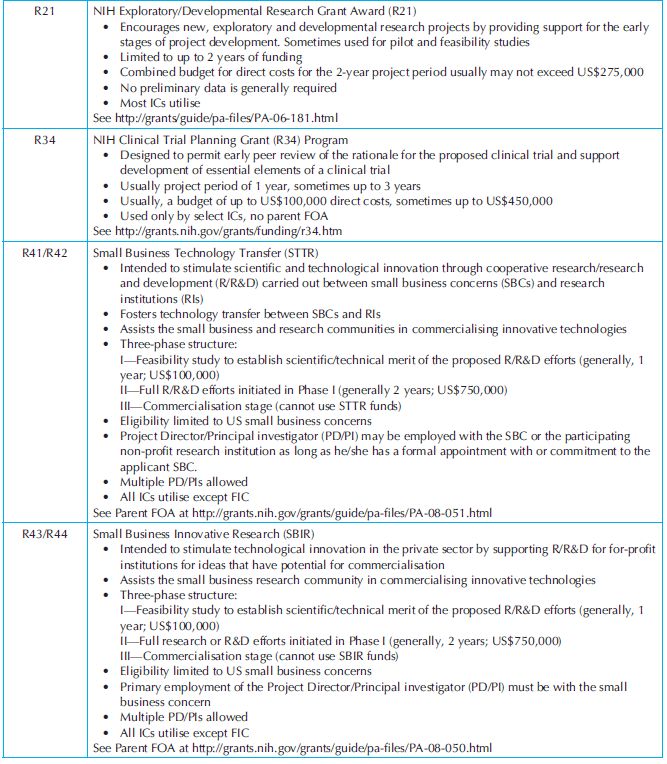
Research grants: Frequently used research grant programmes are presented in Table 34.6. Most NIH’s institutes and centres (ICs) participate in these grant programmes. R-series grants or awards for research projects make up the largest category of activities funded by the NIH (~60% of the total NIH extramural grants). They fund research projects rather than programmes. R-series grants are further divided into several subcategoires; R01 is the traditional research grant supporting research initiated by investigators associated with research institutions. Research institutions typically are responsible for providing facilities necessary to conduct the research proposed by the investigator and are accountable for the grant funds. R03 or ‘small research grants’ supports small research project such as pilot or feasibility studies and secondary analysis of existing data. R41 or R44 are associated with the Small Business Innovation Research and Technology Transfer grants (SBIR and STTR).
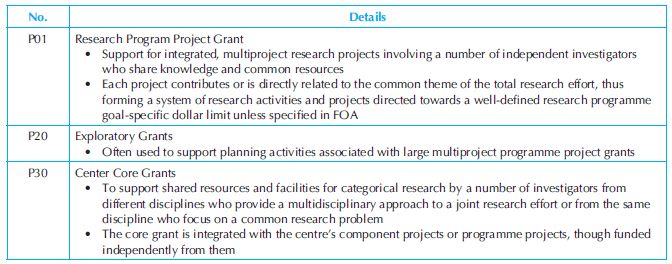
Programme projects/centre grants: Some of the most frequently used programme projects and centre grants are presented in Table 34.7. Programme project/centre grants are large, multi-project efforts that generally include a diverse array of research activities. NIH Institutes and Centres issue funding opportunity announcements to indicate their interest in funding this type of programme. P-series awards can be P01 grants to support multidisciplinary or multifaceted research programmes that have a focused theme.
Table 34.7. Types of NIH’s programme projects/centre grants

Resource grants: Some of the more frequently used types of grant programmes that provide research-related support or access to resources are presented in Table 34.8. However, this list is by no means exhaustive.
Table 34.8. Common types of research programmes

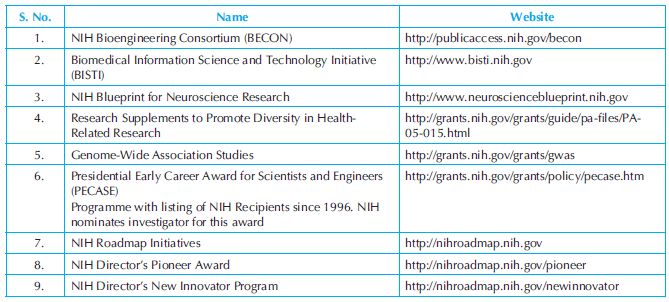
Trans-NIH programmes: NIH supports a variety of broad-reaching programmes that are trans-NIH in nature and these are presented in Table 34.9.
Career Development Awards (K series) [http://grants1.nih.gov/training/careerdevelopmentawards.htm]: The NIH provides several Career Development Awards (K awards) that individuals with a research or medical doctorate should consider. Most of these awards support individuals who have accepted or are ready for a faculty position. There are several career awards including the following:
- Mentored Research Scientist Development Award (K01)
- Independent Scientist Award (K02)
- Senior Scientist Award (K05)
- Academic Career Award (K07)
- Career Enhancement Award for Stem Cell Research (K18)
- Career Transition Award (K22)
- Mentored Quantitative Research Career Development Award (K25)
- Midcareer Investigator Award in Mouse Pathobiology Research (K26)
The main purpose of the K01 programme is to provide support and ‘protected time’ (3–5 years) for an intensive, supervised career development experience in the biomedical, behavioral or clinical sciences leading to research independence. Awards are not renewable, nor are they transferable from one principal investigator to another.
Postdoctoral Fellowships and National Research Service Awards (NRSA; http://grants.nih.gov/training/nrsa.htm): The NIH supports postdoctoral and research training to individuals with or working on a research doctorate or health-professional doctorate. Individual fellowships and institutional research training grants are available from the NIH.
The Ruth L. Kirschstein National Research Service Awards (usually referred to as NRSA) are a family of grants provided by the US NIH for training researchers in behavioural and health sciences. They are a major and highly prestigious source of funding for doctoral and postdoctoral students. NRSA Awards are mostly given to students working on a PhD, an MD or other medical degree, or to individuals who have just earned one of these degrees and are beginning their careers. The NRSA programme also provides institutions with training grants that can be used to fund one or more students. NRSA grants are notable for their flexibility: postdoctoral students can propose to work at any university, and the only requirement is that they commit to at least 1 year of research in their field following their first year of funding.
Small Business Innovation Research Grants (SBIR; http://grants1.nih.gov/grants/funding/sbir.htm): The Small Business Innovation research programme is a set-aside programme (2.5% of an agency’s extramural budget) for domestic small business concerns to engage in R/R&D that has the potential for commercialisation.
Eligibility for NIH grants: NIH announces availability of funds for grant programmes by issuing FOAs in the NIH Guide for Grants and Contracts and on Grants.gov. Parent announcements, programme announcements and requests for applications are all types of FOA. Each type of NIH grant programme has its own set of eligibility requirements. Applicants can find eligibility information in Section III of each FOA. While the PI conceives and writes the application, NIH recognises the applicant institution as the grantee for most grant types. NIH supports scientists at various stages in their careers, from pre-doctoral students on research training grants to investigators with extensive experience who run large research centres. NIH is committed to supporting new investigators. Reviewers give new investigators special consideration, and NIH has programmes targeted specifically for this population.
Generally, PIs and other personnel supported by NIH research grants are not required to be US citizens; however, some NIH programmes/mechanisms have a citizenship requirement. Any citizenship requirement will be stated in the PA or RFA. In general, domestic or foreign, public or private, non-profit or for-profit organisations are eligible to receive NIH grants. NIH may limit eligibility for certain types of programmes, such as limitations on the participation of foreign entities or programmes for which only small businesses are eligible applicants.
In general, foreign institutions and international organisations, including public or private non-profit or for-profit organisations, are eligible to apply for research project grants. Foreign institutions and international organisations are not eligible to apply for Kirschstein-NRSA institutional research training grants, programme project grants, centre grants, resource grants, SBIR/STTR grants or construction grants. However, some activity codes, such as programme project grants (P01), may support projects awarded to a domestic institution with a foreign component. Thus, foreign applicants are strongly encouraged to review the Eligibility section of the FOA to determine whether their non-domestic (non-US) entity (foreign organisation) is eligible to respond to that particular FOA.
Submitting grant application: The majority of competing applications now require electronic application submission. The FOA to which you are applying will identify whether you must submit electronically or use paper submission. Note that paper submissions require use of the PHS 398 application form, while electronic submission requires the SF424 (R&R) application. Make sure to read the instructions in FOA and the application guide to determine submission requirements.
Submission dates. The announcements provide discrete application deadlines or refer to NIH’s standard due dates. The standard application dates are as follows:
- Cycle I: 25th January
- Cycle II: 25th May
- Cycle III: 25th September.
Generally, it takes about 12 months from submission to grant award, including the scientific merit review (4 months), advisory council review (4 months) and processing of project award notice and transfer of funds to the award institution (4 months).
Grant review and award: The NIH awards funds to research performers based on peer review by researchers with active grant awards. Grant proposals are received and processed by the NIH’s Center for Scientific Review which consists of several study sections and advisory panels. Each study section (organised thematically or specialty) is run by the Scientific Review Officer (SRO) with the help of expert scientific reviewers. Each grant proposal is rigorously reviewed and written critique is provided to the applicant along with a numerical priority score. The proposal is judged based on five standard scientific criteria: (i) significance, (ii) technical approach, (iii) innovation, (iv) investigator and (v) environment. Each proposal is reviewed by at least three reviewers. Before review meeting, assigned reviewers and discussants will score applications on the five review criteria, and at the meeting, discussed applications (typically top 50% proposals) will receive an overall score that is converted into a priority score. The priority scores then will be percentiled against the appropriate base. The new scoring system will utilise a 9-point rating scale (from 1 being ‘exceptional’ to 9 being ‘poor’).
Grant applications submitted to the NIH are evaluated on the basis of a process that is fair, equitable, timely and conducted in a manner free of bias. The NIH follows a dual peer-review system. The first level of review is carried out by a Scientific Review Group (SRG) composed primarily of non-federal scientists who have expertise in relevant scientific disciplines and current research areas. The second level of review is performed by Institute and Center (IC) National Advisory Councils or Boards. Councils are composed of both scientific and lay persons chosen for their expertise, interest or activity in matters related to health and disease. Only applications that are favourably recommended by both the SRG and the Advisory Council may be recommended for funding. Priority scores reflect the relative strengths and weaknesses of an application, with the lowest scores indicating the highest level of merit (100–150 being ‘outstanding’) while higher scores (≥200) are acceptable but may not be funded.
The PI gets a Summary Statement from the NIH Program Director. The summary statement is prepared by the SRO of the study section and is based on the reviewers’ reports and the discussion of all the study section members. The summary statement contains the priority score and written critiques from the study section discussion, primary and secondary reviewer; sometimes there is also a tertiary reviewer. If the proposal fails to receive fundable score, the PI is given an opportunity to revise and resubmit the proposal based on the comments in the summary statement.
What does NIH look for in a grant application? Because NIH comprises 24 different grant-awarding ICs which provide funding, there is no simple answer to this question. However, if you talk to an NIH Program Official about your idea or potential research project, you can get excellent feedback if you are on the right track to receiving NIH funding. Specifically, the NIH encourages projects that have the three following qualities:
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree