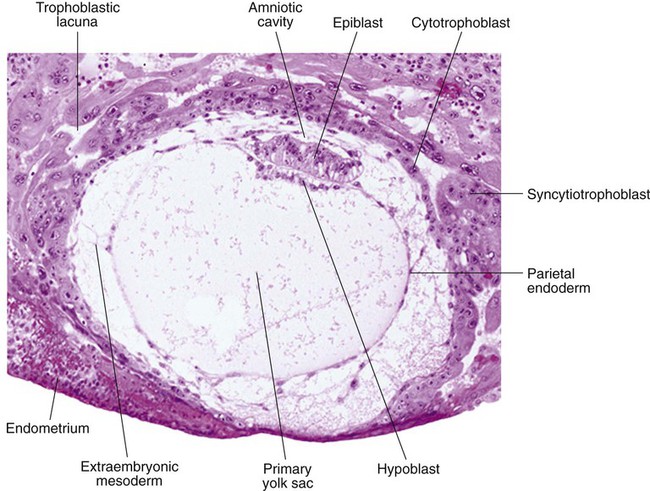
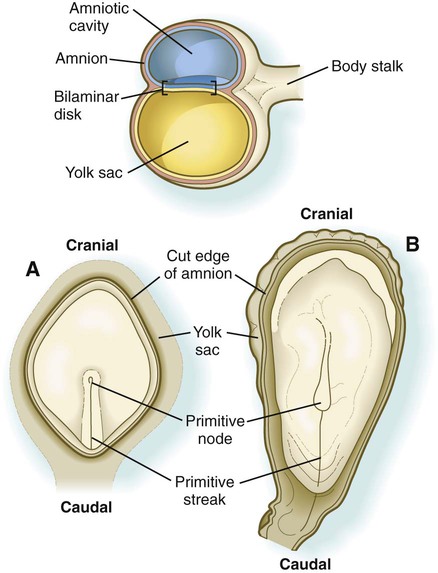
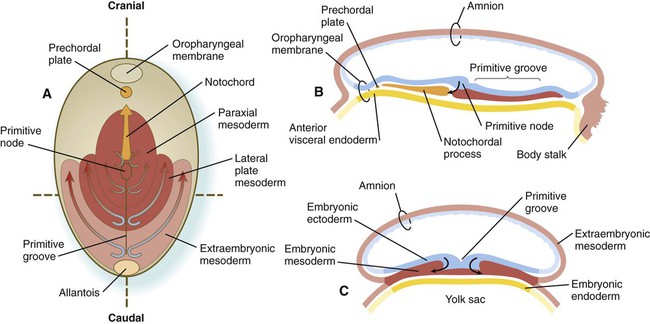
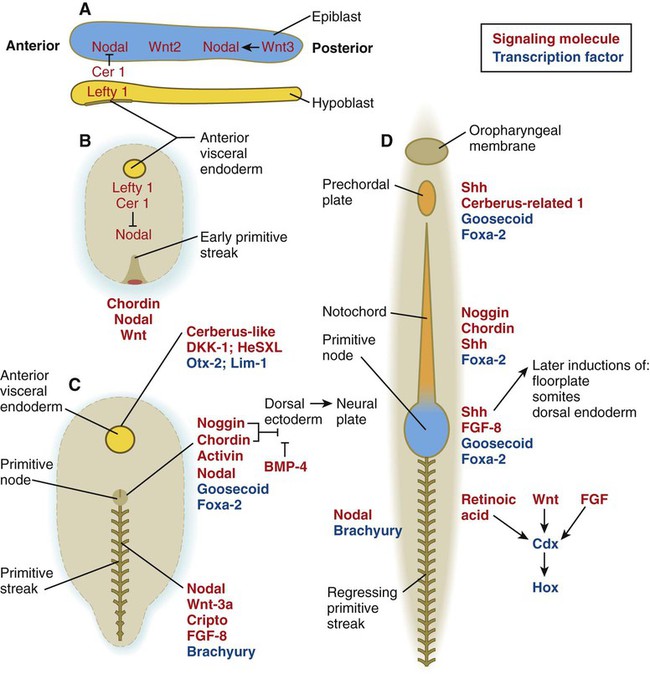
Chapter 5 As it is implanting into the uterine wall, the embryo undergoes profound changes in its organization. Up to the time of implantation, the blastocyst consists of the inner cell mass, from which the body of the embryo proper arises, and the outer trophoblast, which represents the future tissue interface between the embryo and mother. Both components of the blastocyst serve as the precursors of other tissues that appear in subsequent stages of development. Chapter 3 discusses the way in which the cytotrophoblast gives rise to an outer syncytial layer, the syncytiotrophoblast, shortly before attaching to uterine tissue (see Fig. 3.18). Not long thereafter, the inner cell mass begins to give rise to other tissue derivatives as well. The subdivision of the inner cell mass ultimately results in an embryonic body that contains the three primary embryonic germ layers: the ectoderm (outer layer), mesoderm (middle layer), and endoderm (inner layer). The process by which the germ layers are formed through cell movements is called gastrulation. Just before the embryo implants into the endometrium early in the second week, significant changes begin to occur in the inner cell mass and in the trophoblast. As the cells of the inner cell mass become rearranged into an epithelial configuration, sometimes referred to as the embryonic shield, a thin layer of cells appears ventral to the main cellular mass (see Fig. 3.18). The main upper layer of cells is known as the epiblast, and the lower layer is called the hypoblast, or primitive endoderm (Fig. 5.1). How the hypoblast forms in human embryos is not understood, but studies on mouse embryos have shown that as early as the 64-cell stage, some cells of the inner cell mass express the transcription factor nanog, whereas others express Gata 6. These cells are arranged in a salt and pepper pattern within the inner cell mass (Fig. 5.2A). The nanog-expressing cells represent the precursors of the epiblast, and those expressing Gata 6 will become the hypoblast. The basis for the differentiation of these two distinct precursor cell types is not completely understood, but according to the “time inside–time outside” hypothesis, those cells that enter the inner cell mass earliest are biased to express nanog, which perpetuates their pluripotency. Possibly because of the influence of fibroblast growth factor-4 (FGF-4), secreted by these first arrivals to the inner cell mass, later immigrants are then biased to express Gata 6. The Gata 6–expressing cells produce molecules that increase their adhesive properties, as well as their mobility, and they make their way to the lower surface of the inner cell mass to form a thin epithelium, the hypoblast. Those Gata 6 cells that fail to reach the surface of the inner cell mass undergo apoptosis (cell death). The nanog-expressing cells of the inner cell mass also assume an epithelial configuration as they form the epiblast. Between the epiblast and hypoblast a basal lamina forms. A small group of hypoblast cells that becomes translocated to the future anterior end of the embryo (called anterior visceral endoderm by mouse embryologists) has been shown to possess remarkable signaling powers. The cells first secrete the signaling molecules, lefty-1 and Cerberus-1 (Cer-1), which inhibit the activity of the signaling molecules, nodal and Wnt, in the overlying epiblast but allow nodal and Wnt-3 expression in the posterior epiblast (see Fig. 5.8A). (Nodal signaling from the posterior epiblast stimulates the initial formation of the anterior visceral endoderm.) This represents the first clear expression of anteroposterior polarity in the embryo. It also forms two signaling domains within the early embryo. The anterior visceral endoderm soon begins to induce much of the head and forebrain and inhibits the formation of posterior structures. In the posterior part of the epiblast, nodal signaling activity stimulates the formation of the primitive streak (see next section), which is the focal point for gastrulation and germ layer formation. After the hypoblast has become a well-defined layer, and the epiblast has taken on an epithelial configuration, the former inner cell mass is transformed into a bilaminar disk, with the epiblast on the dorsal surface and the hypoblast on the ventral surface. The epiblast contains the cells that make up the embryo itself, but extraembryonic tissues also arise from this layer. The next layer to appear after the hypoblast is the amnion, a layer of extraembryonic ectoderm that ultimately encloses the entire embryo in a fluid-filled chamber called the amniotic cavity (see Chapter 7). Because of the paucity of specimens, the earliest stages in the formation of the human amnion and amniotic cavity are not completely understood. Studies on primate embryos indicate that a primordial amniotic cavity first arises by cavitation (formation of an internal space) within the pre-epithelial epiblast; it is covered by cells derived from the inner cell mass (see Fig. 5.2). According to some investigators, the roof of the amnion then opens, thus exposing the primordial amniotic cavity to the overlying cytotrophoblast. Soon thereafter (by about 8 days after fertilization), the original amniotic epithelium reforms a solid roof over the amniotic cavity. While the early embryo is still sinking into the endometrium (about 9 days after fertilization), cells of the hypoblast begin to spread and line the inner surface of the cytotrophoblast with a continuous layer of extraembryonic endoderm called parietal endoderm (Fig. 5.3; see Fig. 5.2). When the endodermal spreading is completed, a vesicle called the primary yolk sac has taken shape (see Fig. 3.18C). At this point (about 10 days after fertilization), the embryo complex constitutes the bilaminar germ disk, which is located between the primary yolk sac on its ventral surface and the amniotic cavity on its dorsal surface (Fig. 5.4). Shortly after it forms, the primary yolk sac becomes constricted, forming a secondary yolk sac and leaving behind a remnant of the primary yolk sac (see Figs. 3.18D and 5.2F). Starting at about 12 days after fertilization, another extraembryonic tissue, the extraembryonic mesoderm, begins to appear (see Fig. 5.2). The first extraembryonic mesodermal cells seem to arise from a transformation of parietal endodermal cells. These cells are later joined by extraembryonic mesodermal cells that have originated from the primitive streak. The extraembryonic mesoderm becomes the tissue that supports the epithelium of the amnion and yolk sac and the chorionic villi, which arise from the trophoblastic tissues (see Chapter 7). The support supplied by the extraembryonic mesoderm is not only mechanical, but also trophic because the mesoderm serves as the substrate through which the blood vessels supply oxygen and nutrients to the various epithelia. At the end of the second week, the embryo consists of two flat layers of cells: the epiblast and the hypoblast. As the third week of pregnancy begins, the embryo enters the period of gastrulation, during which the three embryonic germ layers form from the epiblast (see Fig. 5.1). The morphology of human gastrulation follows the pattern seen in birds. Because of the large amount of yolk in birds’ eggs, the avian embryo forms the primary germ layers as three overlapping flat disks that rest on the yolk, similar to a stack of pancakes. Only later do the germ layers fold to form a cylindrical body. Although the mammalian egg is essentially devoid of yolk, the morphological conservatism of early development still constrains the human embryo to follow a pattern of gastrulation similar to that seen in reptiles and birds. Because of the scarcity of material, even the morphology of gastrulation in human embryos is not known in detail. Nevertheless, extrapolation from avian and mammalian gastrulation can provide a reasonable working model of human gastrulation. Gastrulation begins with the formation of the primitive streak, a linear midline condensation of cells derived from the epiblast in the posterior region of the embryo through an induction by cells at the edge of the embryonic disk in that region (see Fig. 5.4). Members of the transforming growth factor-β (TGF-β) and Wnt families of signaling molecules have been identified as likely inducing agents. Initially triangular, the primitive streak soon becomes linear and elongates, largely through a combination of proliferation and migration, as well as internal cellular rearrangements, called convergent-extension movements. With the appearance of the primitive streak, the anteroposterior (craniocaudal) and right-left axes of the embryo can be readily identified (see Fig. 5.4). The primitive streak is a region where cells of the epiblast converge in a well-defined spatial and temporal sequence. As cells of the epiblast reach the primitive streak, they change shape and pass through it on their way to forming new layers beneath (ventral to) the epiblast (Fig. 5.5C). Marking studies have shown that cells entering the primitive streak form distinct lineages as they leave. The most posterior cells both to enter and leave the streak as it is beginning to elongate form the extraembryonic mesoderm lining the trophoblast and yolk sac, as well as that forming the blood islands (see Fig. 6.19). Another wave of mesoderm, arising later and more anteriorly in the primitive streak, forms the paraxial, lateral plate, and cardiac mesoderm. A final wave, which enters and leaves the anteriormost end of the primitive streak, gives rise to midline axial structures (the notochord, the prechordal plate, and the primitive node itself) and also the embryonic endoderm. The composite results of such marking experiments are organized into fate maps, such as that illustrated in Figure 5.5A. The endodermal precursor cells that pass through the anterior primitive streak largely displace the original hypoblast, but research has shown that some of the original hypoblastic cells become integrated into the newly forming embryonic endodermal layer. The displaced hypoblastic cells form extraembryonic endoderm. The movement of cells through the primitive streak results in the formation of a groove (primitive groove) along the midline of the primitive streak. At the anterior end of the primitive streak is a small but well-defined accumulation of cells, called primitive node, or Hensen’s node.* This structure is of great developmental significance because, in addition to being the major posterior signaling center of the embryo (Box 5.1), it is the area through which cells migrate in a stream toward the anterior end of the embryo. These cells, called mesendoderm, soon segregate into a rodlike mesodermal notochord and the endodermal dorsal wall of the forming gut. Anterior to the notochord is a group of mesodermal cells called the prechordal plate (see Fig. 5.5A and B). (The important functions of the notochord and prechordal plate are discussed on p. 80.)
Formation of Germ Layers and Early Derivatives
Two-Germ-Layer Stage
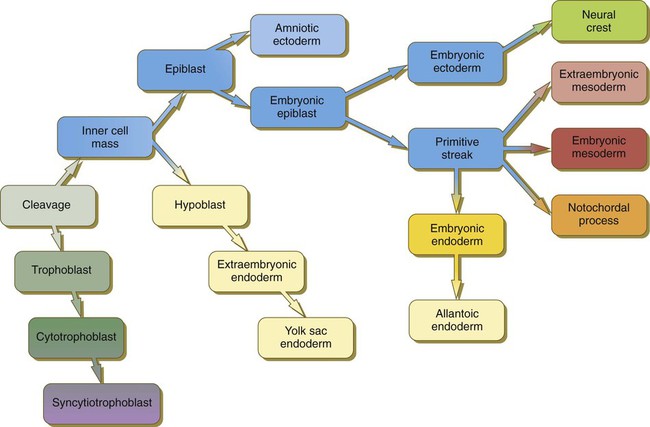
(Note: The colors in the boxes are found in all illustrations involving the embryonic and extraembryonic germ layers.)
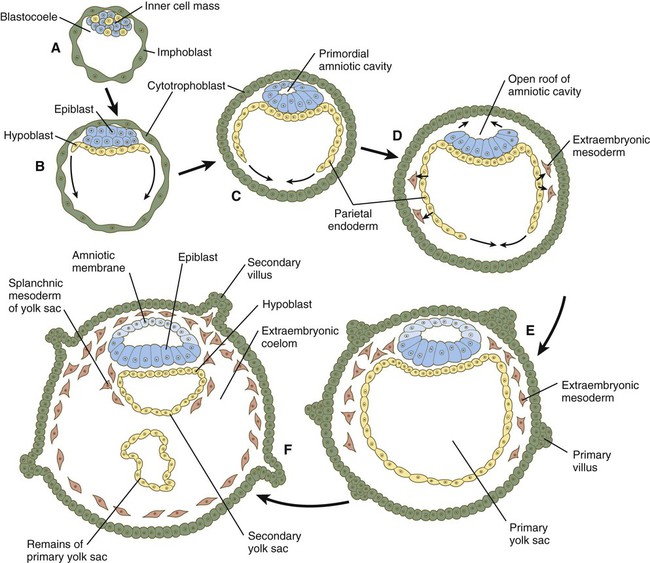
The syncytiotrophoblast is not shown. A, Late blastocyst. Within the inner cell mass, blue nanog-expressing pre-epiblastic cells and yellow Gata 6–expressing prehypoblastic cells are mixed in a salt and pepper pattern. B, Beginning of implantation at 6 days. The hypoblast has formed and is beginning to spread beneath the cytotrophoblast as the parietal endoderm. C, Implanted blastocyst at 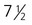 days. D, Implanted blastocyst at 8 days. E, Embryo at 9 days. F, Late second week.
days. D, Implanted blastocyst at 8 days. E, Embryo at 9 days. F, Late second week.
Gastrulation and the Three Embryonic Germ Layers
Formation of Germ Layers and Early Derivatives