Chapter 63 • Monitor renal, hepatic, and hematopoietic function during prolonged therapy. • Patients on warfarin anticoagulation should be monitored closely for prothrombin times and INR at baseline—daily for the first week of therapy, and weekly thereafter. The patient should be instructed to report immediately any sign of bleeding. • Patients on any theophylline product (e.g., theophylline, oxtriphylline, aminophylline) should have serum theophylline levels monitored because theophylline clearance may be decreased with concomitant fluoroquinolone use. • Evaluate renal and hepatic function at baseline and every 6 weeks if therapy is to be continued. • Monitor hematology parameters periodically for evidence of leukopenia, hemolytic anemia, and thrombocytopenia. • Monitor for CNS side effects such as headache, weakness, shaking, dizziness, drowsiness, and confusion.
Fluoroquinolones
Class
Generic Name
Trade Name
Fluoroquinolones
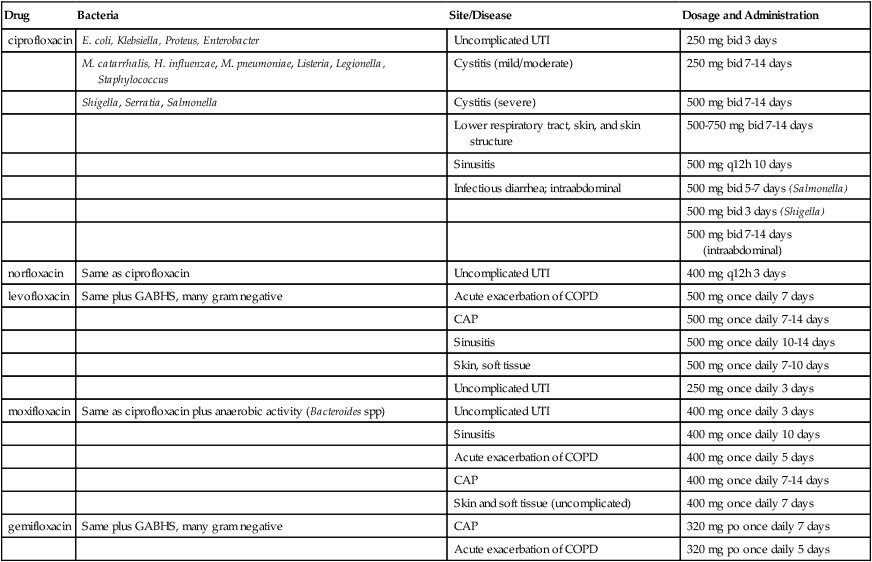
ciprofloxacin ![]()
Cipro
norfloxacin
Noroxin
ofloxacin
Floxin
Fluoroquinolones with enhanced activity against Streptococcus pneumoniae
levofloxacin
Levaquin
moxifloxacin
Avelox
gemifloxacin
Factive

Treatment Principles
How to Monitor
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


Fluoroquinolones
Only gold members can continue reading. Log In or Register to continue