DRUG CLASSES
Electrolytes
• Calcium
• Magnesium
• Potassium
• Sodium
Alkaline/acidifying agents
PHARMACOLOGY IN PRACTICE

Alfredo Garcia is a 55-year-old man being seen in the clinic for an upper respiratory infection. During the intake assessment, you find that his blood pressure is high and he continues to complain of “heartburn.” Mr. Garcia attempted to use home remedies (e.g., drinking cream) to decrease acid production. You have taught him about ulcer treatment, yet his heartburn persists. He continues to rely on home remedies, which are causing other problems. Mr. Garcia’s weight is up, and looking at his legs, you note +2 pitting edema bilaterally. While reading the chapter, you will learn about some of the problems that can happen with electrolyte imbalances.
Over 50% of our body is made up of fluid. It gives our cells shape, acts as a cushion by filling the void between cells, and travels throughout our bodies with elements to help cells work or eliminate waste. The electrolytes also play important roles in our daily function. This chapter offers information about our fluids and electrolytes as well as basics on parenteral (IV) therapy. The clinical setting where nurses work determines the role you will play in IV therapy. Who starts the IV, monitors it, and accesses the line for medication administration is different in each setting. No matter where you practice nursing, it is important to understand the basic concepts of fluid and electrolyte balance.
Fluid makes up a major portion of our body tissues. When individuals become ill, they may lose excess amounts of fluid or not feel well enough to maintain the daily intake of fluid needed for basic survival. Without fluids, the body’s ability to transport oxygen and nutrients to the various tissues is compromised. Individual cells or organs are not able to function and the person becomes even more ill. Various solutions are used in the management of body fluids. These solutions are used when the body cannot sustain the fluid or electrolyte balance needed for normal functioning.
Parenteral Management of Body Fluids
Parenteral administration includes the injecting of drugs or solutions directly into a vein. In the acute care or urgent care setting, solutions used to manage body fluids are usually administered IV. Intravenous replacement solutions are used for the following:
• As a parenteral source of electrolytes, calories, or water for hydration
• To facilitate nutrition and maintain electrolyte balance when the patient cannot eat
• As a method to deliver drugs when a less invasive method is not suitable due to drug pharmacokinetics or patient status
Establishing Intravenous Access
All facilities have specific policies and procedures for cleaning, access, and stabilization of IV sites. Basic steps are outlined in this section. When selecting a vein for IV access, the nondominant arm is typically selected and inspected, and a site is often picked at the most distal point. Larger, more proximal veins may be selected, depending on the need for the IV therapy. You should ask the patient about previous IV therapy. The patient can frequently tell you if a specific site gives providers trouble when access is attempted. This can save time and reduce the pain of an unsuccessful attempt when the patient’s knowledge of previous care is considered.
When a site has been selected for venipuncture, place a tourniquet above the selected vein. It is important to tighten the tourniquet so that venous blood flow is blocked but arterial blood flow is not. Cleanse the site according to facility policy. The vein fills (distends) and then the skin is pulled taut (to anchor the vein and the skin) and the needle is inserted into the vein bevel up, and at a low angle to the skin. Blood should immediately flow into the syringe if the needle is properly inserted into the vein. If the IV is being placed for a period of time, a device with a cannula is used. With this device, the needle is then removed, and a flexible cannula remains in the vein. The cannula is secured in place, and the device is ready for administration of drugs or fluids.
Performing a venipuncture requires practice. A suitable vein for venipuncture may be hard to find, and some veins are difficult to enter. Never repeatedly and unsuccessfully attempt a venipuncture. Depending on clinical judgment, two unsuccessful attempts on the same patient warrant having a more skilled individual attempt the procedure.
Maintaining Intravenous Access
Intermittent Intravenous Access
Fluids, electrolytes, and drugs are given by direct IV push, intermittent infusion, or continuous infusion. When the direct IV method or intermittent infusion is used, the cannula that stays in the vein, an adapter, and small tubing are called a heparin (or saline) lock, and it is secured to the arm (Fig. 54.1). The device allows for a dose to be given directly into a vein or via intermittent IV administration without having to maintain an infusion. A lock also gives the patient the ability to move about more freely without cumbersome IV lines or machines to impede activities such as ambulation.
To maintain the patency of the lock, a solution of saline or dilute heparin may be ordered for injection into the heparin lock before and after the administration of a drug administered via the IV route. This is called a lock flush. The flush solution aids in preventing small clots from obstructing the cannula of the IV administration set. Patients may hear providers refer to the device as both a saline and a heparin lock. The primary health care provider or institutional policy dictates the use and strength of heparin lock flush solution. It is important to know the policies of your institution to be sure that you access the device with the appropriate solution. Should the policy of your institution be the use of heparin, be sure to prevent incompatibility of heparin with other drugs, and flush with sterile normal saline solution before and after any drug is given through the IV line.
 NURSING ALERT
NURSING ALERT
Do not mix multiple IV medications in a syringe (e.g., benzodiazepines and furosemide) without consulting the clinical pharmacist about compatibility. When injecting multiple drugs sequentially, always flush thoroughly and monitor the IV site for phlebitis and/or thrombosis.
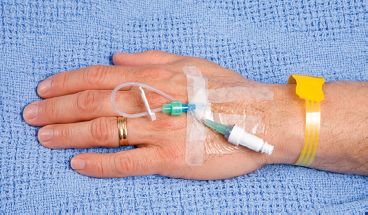
Figure 54.1 IV access (saline/heparin lock) for intermittent infusions or direct administration of drugs.
Figure 54.2 The nurse sets the electronic IV device to deliver a specific amount of IV fluid.
Continuous Infusion Access
Continuous infusion involves a continual flow of fluid into a vein for replacement purposes and continuous or frequent drug administration. Electronic infusion devices monitor the rate and flow of intravenous fluids (Fig. 54.2). The purpose of the device is to monitor the flow of the intravenous solution. An alarm is set to sound if the rate of infusion is more or less than the preset rate. These machines are classified as either infusion controllers or infusion pumps. The primary difference between the two is that an infusion pump adds pressure to the infusion, whereas an infusion controller does not.
When any problem is detected by the device, an alarm is activated to alert the nurse. Controllers and pumps have detectors and alarms that sound when various problems occur, such as air in the line, an occlusion, low battery, completion of an infusion, or an inability to deliver the preset rate.
 NURSING ALERT
NURSING ALERT
Use of an infusion pump or controller still requires nursing supervision and frequent monitoring of the IV infusion.
It is important to monitor frequently for signs of infiltration (the collection of fluid into tissues), such as edema or redness at the site. Infiltration can progress rapidly, because with the increased pressure the infusion will not slow until considerable edema has occurred. Careful monitoring of the pump or controller is also necessary to make sure the flow rate is correct.
When the patient needs fluid replacement and drugs are to be administered, a secondary line is established using the IV route. Intermittent IV infusion is given using “Y” tubing while another solution is being given on a continuous basis. When this method is used, depending on the machine you may have to clamp off the IV fluid given on a continuous basis while the drug is allowed to infuse or the two solutions may infuse at the same time.
Calculating Intravenous Flow Rates
When fluid replacement is indicated, the amount (or volume) of fluid to be administered is ordered over a specified time period, such as 125 mL/hr or 1000 mL over 8 hours. Many infusion machines work by setting the volume of fluid to be infused over a specific time period. If the IV is not infused through a machine or if the machine monitors the drip rate, then the IV flow rate must be calculated.
The IV flow rate is obtained by counting the number of drops in the drip chamber and using the amount of fluid in each drop to calculate the volume. Drip chambers on the various types of IV fluid administration sets vary. Some deliver 15 drops/mL and others deliver more or less than this number. This is called the drop factor. The drop factor (number of drops/mL) is given on the package containing the drip chamber and IV tubing. Three methods for determining the IV infusion rate follow. Methods 1 and 2 can be used when the known factors are the total amount of solution, the drop factor, and the number of hours over which the solution is to be infused.
Method 1
Step 1. Total amount of solution ÷ number of hours = number of mL/hr
Step 2. mL/hr ÷ 60 min/hr = number of mL/min
Step 3. mL/min × drop factor = number of drops/min
Example
1000 mL of an IV solution is to infuse over a period of 8 hours. The drop factor is 15.
Step 1. 1000 mL ÷ 8 hours = 125 mL/hr
Step 2. 125 mL ÷ 60 minutes = 2.08 mL/min
Step 3. 2.08 × 15 = 31.2 or (31 to 32) drops/min
Method 2
Step 1. Total amount of solution ÷ number of hours = number of mL/hr
Step 2. mL/hr × drop factor ÷ 60 = number of drops/min
Example
1000 mL of an IV solution is to infuse over a period of 6 hours. The drop factor is 10.
Step 1. 1000 mL ÷ 6 hours = 166.6 mL/hr
Step 2. 166.6 × 10 ÷ 60 = 26.66 or (26 to 27) drops/min
Method 3
This method may be used when the desired amount of solution to be infused in 1 hour is known or written as a physician’s order.
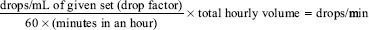
Example
If a set delivers 15 drops/min and 240 mL is to be infused in 1 hour:

Fluid Overload
One problem commonly associated with all solutions administered by the parenteral route is fluid overload, that is, the administration of more fluid than the body is able to handle.
The term fluid overload (also circulatory overload) describes a condition when the body’s fluid requirements are met and the administration of fluid occurs at a rate that is greater than the rate at which the body can use or eliminate the fluid. Thus, the amount of fluid and the rate of administration of fluid that will cause fluid overload depend on several factors, such as the patient’s cardiac status and adequacy of renal function. The signs and symptoms of fluid overload are listed in Display 54.1.
Display 54.1 Signs and Symptoms of Fluid Overload
• Headache
• Weakness
• Blurred vision
• Behavioral changes (confusion, disorientation, delirium, drowsiness)
• Weight gain
• Isolated muscle twitching
• Hyponatremia
• Rapid breathing
• Wheezing
• Coughing
• Rise in blood pressure
• Distended neck veins
• Elevated central venous pressure
• Convulsions
Solutions Used in the Management of Body Fluids
The next section gives a brief overview of the following IV replacement solutions: electrolytes, blood products and expanders, and total parenteral nutrition (TPN). One of the primary purposes of administering an IV solution is to provide proper electrolyte balance in the body.
ELECTROLYTES
An electrolyte is an electrically charged particle essential to the normal functioning of all cells. Intracellular and extracellular fluids have specific chemical compositions of electrolytes. Major electrolytes in intracellular fluid include:
• Potassium
• Magnesium
Major electrolytes in extracellular fluid include:
• Sodium
• Calcium
Electrolytes circulate in the blood at specific levels, where they are available for use when needed by the cells. An electrolyte imbalance occurs when the concentration of an electrolyte in the blood is either too high or too low. In some instances, an electrolyte imbalance may be present without an appreciable disturbance in fluid balance. An electrolyte imbalance can profoundly affect a patient’s physiologic functioning, the body’s water distribution, neuromuscular activity, and acid-base balance. An electrolyte imbalance can occur from any disorder that alters electrolyte levels in the body’s fluid compartments. An imbalance can also occur from vomiting, surgery, diagnostic tests, or drug administration. For example, a patient taking a diuretic is able to maintain fluid balance by an adequate oral intake of water, which replaces the water lost through diuresis. However, the patient is likely to be unable to replace the potassium that is also lost during diuresis. When the potassium concentration in the blood is too low, as may occur with the administration of a diuretic, an imbalance may occur that requires the addition of potassium. Electrolyte replacement drugs are inorganic or organic salts that increase deficient electrolyte levels that help to maintain homeostasis. Commonly used electrolyte replacement drugs are listed in the Summary Drug Table: Electrolytes.
INTRACELLULAR ELECTROLYTES
Action and Uses
Potassium (K+)
Potassium is the major electrolyte in intracellular fluid and must be consumed daily because it cannot be stored. It is necessary for the transmission of impulses; the contraction of smooth, cardiac, and skeletal muscles; and other important physiologic processes. Potassium may be given to correct hypokalemia (low blood potassium) resulting from increased potassium excretion or depletion. Examples of causes of hypokalemia are a marked loss of GI fluids (severe vomiting, diarrhea, nasogastric suction, draining intestinal fistulas), diabetic acidosis, marked diuresis, severe malnutrition, use of a potassium-depleting diuretic, excess antidiuretic hormone, and excessive urination. Potassium as a drug is available as potassium chloride (KCl) and potassium gluconate, and is measured in milliequivalents (mEq)—for example, 40 mEq in 20 mL or 8-mEq controlled-release tablet.
Magnesium (Mg++)
Magnesium plays an important role in the transmission of nerve impulses. It is also important in the activity of many enzyme reactions, such as carbohydrate metabolism. Magnesium sulfate (MgSO4) is used as replacement therapy in hypomagnesemia. Magnesium is also used in the prevention and control of seizures in obstetric patients with pregnancy-induced hypertension (PIH; also referred to as eclampsia and preeclampsia). It may also be added to TPN mixtures.
Adverse Reactions, Contraindications, Precautions, and Interactions
Potassium (K+)
Nausea, vomiting, diarrhea, abdominal pain, and phlebitis have been seen with oral and IV administration of potassium. Adverse reactions related to hypokalemia or hyperkalemia are listed in Display 54.2.
Potassium is contraindicated in patients who are at risk for hyperkalemia, such as those with renal failure, oliguria, azotemia (the presence of nitrogen-containing compounds in the blood), anuria, severe hemolytic reactions, untreated Addison’s disease, acute dehydration, heat cramps, and any form of hyperkalemia. Potassium is used cautiously in patients with renal impairment or adrenal insufficiency, heart disease, metabolic acidosis, or prolonged or severe diarrhea.
Display 54.2 Signs and Symptoms of Electrolyte Imbalances
Calcium
Normal laboratory values: 4.5–5.3 mEq/L or 9–11 mg/dL*
Hypocalcemia
Hyperactive reflexes, carpopedal spasm, perioral paresthesias, positive Trousseau’s sign, positive Chvostek’s sign, muscle twitching, muscle cramps, tetany (numbness, tingling, and muscular twitching usually of the extremities), laryngospasm, cardiac arrhythmias, nausea, vomiting, anxiety, confusion, emotional lability, convulsions
Hypercalcemia
Anorexia, nausea, vomiting, lethargy, bone tenderness or pain, polyuria, polydipsia, constipation, dehydration, muscle weakness and atrophy, stupor, coma, cardiac arrest
Magnesium
Normal laboratory values: 1.5–2.5 mEq/L or 1.8–3 mg/dL*
Hypomagnesemia
Leg and foot cramps, hypertension, tachycardia, neuromuscular irritability, tremor, hyperactive deep tendon reflexes, confusion, disorientation, visual or auditory hallucinations, painful paresthesias, positive Trousseau’s sign, positive Chvostek’s sign, convulsions
Hypermagnesemia
Lethargy, drowsiness, impaired respiration, flushing, sweating, hypotension, weak to absent deep tendon reflexes
Potassium
Normal laboratory values: 3.5–5 mEq/L*
Hypokalemia
Anorexia, nausea, vomiting, mental depression, confusion, delayed or impaired thought processes, drowsiness, abdominal distention, decreased bowel sounds, paralytic ileus, muscle weakness or fatigue, flaccid paralysis, absent or diminished deep tendon reflexes, weak and irregular pulse, paresthesias, leg cramps, electrocardiographic changes
Hyperkalemia
Irritability, anxiety, listlessness, mental confusion, nausea, diarrhea, abdominal distress, GI hyperactivity, paresthesias, weakness and heaviness of the legs, flaccid paralysis, hypotension, cardiac arrhythmias, electrocardiographic changes
Sodium
Normal laboratory values: 132–145 mEq/L*
Hyponatremia
Cold and clammy skin, decreased skin turgor, apprehension, confusion, irritability, anxiety, hypotension, postural hypotension, tachycardia, headache, tremors, convulsions, abdominal cramps, nausea, vomiting, diarrhea
Hypernatremia
Fever; hot, dry skin; dry, sticky mucous membranes; rough, dry tongue; edema; weight gain; intense thirst; excitement; restlessness; agitation; oliguria or anuria
*These laboratory values may not concur with the normal range of values in all hospitals and laboratories. The hospital policy manual or laboratory values sheet should be consulted for the normal ranges of all laboratory tests.
Concurrent use of potassium with angiotensin-converting enzyme (ACE) inhibitors may result in an elevated serum potassium level. Potassium-sparing diuretics and salt substitutes used with potassium can produce severe hyperkalemia. The use of digitalis with potassium increases the risk of digoxin toxicity.
Magnesium (Mg++)
Adverse reactions from magnesium administration are most likely related to overdose and may include flushing, sweating, hypotension, depressed reflexes, muscle weakness, respiratory failure, and circulatory collapse (see Display 54.2). Magnesium is contraindicated in patients with heart block or myocardial damage and in women with PIH during the 2 hours before delivery. Magnesium is a pregnancy category A drug, and there is no increased risk of fetal abnormalities if the agent is used during pregnancy. Nevertheless, caution is used when administering magnesium during pregnancy.
Magnesium is used with caution in patients with renal function impairment. When magnesium is used with alcohol, antidepressants, antipsychotics, barbiturates, hypnotics, general anesthetics, and opioids, an increase in central nervous system depression may occur. Prolonged respiratory depression and apnea may occur when magnesium is administered with the neuromuscular blocking agents. When magnesium is used with digoxin, heart block may occur.
Extracellular Electrolytes
Action and Uses
Sodium (Na+)
Sodium is a major electrolyte in extracellular fluid and is important in maintaining acid-base balance and normal heart action, and in the regulation of osmotic pressure in body cells (water balance). Sodium is administered for hyponatremia (low blood sodium). Examples of causes of hyponatremia are excessive diaphoresis, severe vomiting or diarrhea, excessive diuresis, diuretic use, wound drainage, and draining intestinal fistulas.
Sodium, as sodium chloride (NaCl), may be given IV. A solution containing 0.9% NaCl is called normal saline, and a solution containing 0.45% NaCl is called half-normal saline. Sodium also is available combined with dextrose, such as dextrose 5% and sodium chloride 0.9% (D5NS).
Calcium (Ca++)
Calcium is necessary for the functioning of nerves and muscles, the clotting of blood, the building of bones and teeth, and other physiologic processes. Examples of calcium salts are calcium gluconate and calcium carbonate. Calcium may be given for the treatment of hypocalcemia (low blood calcium), which may be seen in those with parathyroid disease or after accidental removal of the parathyroid glands during surgery of the thyroid gland. Calcium may also be given during cardiopulmonary resuscitation, particularly after open heart surgery, when epinephrine fails to improve weak or ineffective myocardial contractions. Calcium may be used as adjunct therapy of insect bites or stings to reduce muscle cramping, such as occurs with black widow spider bites. Calcium may also be recommended for those eating a diet low in calcium or as a dietary supplement when there is an increased need for calcium, such as during pregnancy.
Combined Electrolyte Solutions
Combined electrolyte solutions are available for oral and IV administration. The IV solutions contain various electrolytes and dextrose. The amount of electrolytes, given as milliequivalents per liter (mEq/L), also varies. The IV solutions are used to replace fluid and electrolytes that have been lost and to provide calories through their carbohydrate content. Examples of IV electrolyte solutions are dextrose 5% with 0.9% NaCl, lactated Ringer’s, and Plasma-Lyte. The primary health care provider selects the type of combined electrolyte solution that will meet the patient’s needs.
Oral electrolyte solutions contain a carbohydrate and various electrolytes. Examples of combined oral electrolyte solutions are Pedialyte and Rehydralyte. Oral electrolyte solutions are most often used to replace lost electrolytes and fluids in conditions such as severe vomiting or diarrhea.
Adverse Reactions, Contraindications, Precautions, and Interactions
Sodium (Na+)
Sodium as the salt (e.g., NaCl) has no adverse reactions except those related to overdose (see Display 54.2). In some instances, excessive oral use may produce nausea and vomiting.
Sodium is contraindicated in patients with hypernatremia or fluid retention, and when the administration of sodium or chloride could be detrimental. Sodium is used cautiously in surgical patients and those with circulatory insufficiency, hypoproteinemia, urinary tract obstruction, heart failure (HF), edema, or renal impairment. Sodium is a pregnancy category C drug and is used cautiously during pregnancy.
Calcium (Ca++)
Irritation of the vein used for administration, tingling, a metallic or chalky taste, and “heat waves” may occur when calcium is given IV. Rapid IV administration (calcium gluconate) may result in bradycardia, vasodilation, decreased blood pressure, cardiac arrhythmias, and cardiac arrest. Oral administration may result in GI disturbances. Administration of calcium chloride may cause peripheral vasodilation, a temporary fall in blood pressure, and a local burning. Display 54.2 lists adverse reactions associated with hypercalcemia and hypocalcemia.
Blood Products And Expanders
Actions and Uses
Blood Plasma
Blood plasma is the liquid part of blood, containing water, sugar, electrolytes, fats, gases, proteins, bile pigment, and clotting factors. Human plasma, also called human pooled plasma, is obtained from donated blood. Although whole blood must be typed and crossmatched because it contains red blood cells carrying blood type and Rh factors, human plasma does not require this procedure. Because of this, plasma can be given in acute emergencies.
Plasma administered IV is used to increase blood volume when severe hemorrhage has occurred and it is necessary partially to restore blood volume while waiting for whole blood to be typed and crossmatched or when plasma alone has been lost, as may be seen in severe burns.
Plasma Protein Fractions
Plasma protein fractions include human plasma protein fraction 5% and normal serum albumin 5% (Albuminar-5, Buminate 5%) and 25% (Albuminar-25, Buminate 25%). Plasma protein fraction 5% is an IV solution containing 5% human plasma proteins. Serum albumin is obtained from donated whole blood and is a protein found in plasma. The albumin fraction of human blood acts to maintain plasma colloid osmotic pressure and as a carrier of intermediate metabolites in the transport and exchange of tissue products. It is critical in regulating the volume of circulating blood. When blood is lost from shock, such as in hemorrhage, there is a reduced plasma volume. When blood volume is reduced, albumin quickly restores the volume in most situations.
Plasma protein fractions are used to treat hypovolemic (low blood volume) shock that occurs as the result of burns, trauma, surgery, and infections, or in conditions where shock is not currently present but likely to occur. As with human pooled plasma, blood type and crossmatch are not needed when plasma protein fractions are given. Adverse reactions are rare when plasma protein fractions are administered, but nausea, chills, fever, urticaria, and hypotensive episodes may occasionally be seen. Plasma proteins are contraindicated in those with a history of allergic reactions to albumin, severe anemia, or cardiac failure; in the presence of normal or increased intravascular volume; and in patients on cardiopulmonary bypass. Plasma protein fractions are used cautiously in patients who are in shock or dehydrated and in those with heart failure or hepatic or renal failure.
Most of these solutions should not be combined with any other solutions or drugs but should be administered alone. Consult the drug insert or other appropriate sources before combining any drug with any plasma protein fraction. Solutions used in the management of body fluids are contraindicated in patients with hypersensitivity to any component of the solution. All solutions used to manage body fluids discussed in this chapter are pregnancy category C drugs and are used cautiously during pregnancy and lactation. No interactions have been reported.
Plasma Expanders
The IV solutions of plasma expanders include hetastarch (Hespan), low–molecular-weight dextran (Dextran 40), and high–molecular-weight dextran (Dextran 70, Dextran 75).
Plasma expanders are used to expand plasma volume when shock is caused by burns, hemorrhage, surgery, and other trauma or for prophylaxis of venous thrombosis and thromboembolism. When used in the treatment of shock, plasma expanders are not a substitute for whole blood or plasma, but they are of value as emergency measures until the latter substances can be used.
Administration of hetastarch, a plasma expander, may be accompanied by vomiting, a mild temperature elevation, itching, and allergic reactions. Allergic reactions are evidenced by wheezing, swelling around the eyes (periorbital edema), and urticaria. Other plasma expanders may result in mild cutaneous eruptions, generalized urticaria, hypotension, nausea, vomiting, headache, dyspnea, fever, tightness of the chest, bronchospasm, wheezing, and, rarely, anaphylactic shock.
Plasma expanders are contraindicated in patients with hypersensitivity to any component of the solution and those with severe bleeding disorders, severe cardiac failure, renal failure with oliguria, or anuria. Plasma expanders are used cautiously in patients with renal disease, HF, pulmonary edema, and severe bleeding disorders. Plasma expanders are pregnancy category C drugs and are used cautiously during pregnancy and lactation. Consult the drug insert or other appropriate sources before combining a plasma expander with another drug for IV administration.
TOTAL PARENTERAL NUTRITION
When normal enteral feeding is not possible or is inadequate to meet an individual’s nutritional needs, IV nutritional therapy or TPN is required. TPN is a method of administering nutrients to the body by an IV route. TPN uses a complex admixture of chemicals combined in a single container. The components of the TPN mixture may include proteins (amino acids), fats, glucose, electrolytes, vitamins, minerals, and sterile water. Products used to meet the IV nutritional requirements of the patient include protein substrates (amino acids), energy substrates (dextrose and fat emulsions), fluids, electrolytes, and trace minerals.
Total parenteral nutrition is used to prevent nitrogen and weight loss or to treat negative nitrogen balance (a situation in which more nitrogen is used by the body than is taken in) in the following situations:
• Oral, gastrostomy, or jejunostomy route cannot or should not be used.
• GI absorption of protein is impaired by obstruction.
• Inflammatory disease or antineoplastic therapy prevents normal GI functioning.
• Bowel rest is needed (e.g., after bowel surgery).
• Metabolic requirements for protein are significantly increased (e.g., in hypermetabolic states such as serious burns, infections, or trauma).
• Morbidity and mortality may be reduced by replacing amino acids lost from tissue breakdown (e.g., renal failure).
• Tube feeding alone cannot provide adequate nutrition.
If a patient’s intake of protein nutrients is significantly less than is required by the body to meet energy expenditures, a state of negative nitrogen balance occurs. The body begins to convert protein from the muscle into carbohydrate for energy to be used by the body. This results in weight loss and muscle wasting. In these situations, traditional IV fluids do not provide sufficient calories or nitrogen to meet the body’s daily requirements. TPN may be administered through a peripheral vein or a central venous catheter in a highly concentrated form to improve nutritional status, establish a positive nitrogen balance, and enhance the healing process. Peripheral TPN is used for relatively short periods (no more than 5 to 7 days) and when the central venous route is not possible or necessary. An example of a solution used in TPN is amino acids with electrolytes. These solutions may be used alone or combined with dextrose (5% or 10%) solutions.
Total parenteral nutrition through a central vein is indicated to promote protein synthesis in patients who are severely hypercatabolic or severely depleted of nutrients, or who require long-term parenteral nutrition. For example, amino acids combined with hypertonic dextrose and IV fat emulsions are infused through a central venous catheter to promote protein synthesis. Vitamins, trace minerals, and electrolytes may be added to the TPN mixture to meet the patient’s individual needs. The daily dose depends on the patient’s daily protein requirement and his or her metabolic state and clinical responses.
TPN is delivered with an infusion pump. The pump infuses a small amount (0.1 to 10 mL/hr) continuously to keep the vein open. Feeding schedules vary; one example is administration of the feeding continuously over a few hours, leveling off the rate for several hours, and then increasing the rate of administration for several hours to simulate a normal set of meal times.
 NURSING ALERT
NURSING ALERT
Hyperglycemia is a common metabolic complication. If an infusion of TPN is given too rapidly it may result in hyperglycemia, glycosuria, mental confusion, and loss of consciousness. Assess blood glucose levels every 4 to 6 hours to monitor for hyperglycemia and guide the dosage of dextrose and insulin (if required). To minimize these complications, the primary health care provider may decrease the rate of administration, reduce the dextrose concentration, or administer insulin.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree



