http://evolve.elsevier.com/McCuistion/pharmacology
Fluid and electrolyte balance is necessary to maintain homeostasis. An equal balance of intake and output helps the human body maintain proper equilibrium within all body systems. Intake and output of water is regulated by the kidneys and the pulmonary system and by hormonal and neural functions. Fluid and electrolyte balance is the foundation upon which nurses make important decisions concerning patient care.
To function normally, body cells must have fluid and electrolytes in the right compartments and in the right amounts. Total body fluid is contained in two major compartments: the intracellular fluid (ICF) is contained within the body’s cells, and the extracellular fluid (ECF) comprises all the fluid outside of the cells. The ECF is further divided into three subcompartments: the interstitial compartment bathes and surrounds the tissue cells; the intravascular compartment contains the plasma and blood vessels; and the transcellular compartment, also known as the third-space, contains mucus and gastrointestinal (GI), cerebrospinal, pericardial, synovial, and ocular fluids. Table 12.1 shows the sum of fluids within all compartments, which constitutes the total body water (TBW). Fluids move freely between the intracellular and extracellular compartments to maintain balance and homeostasis.
The TBW of a 70-kg (154-lb) man is approximately 60% (40 L). This percentage varies with age, gender, and percentage of body fat. Neonates are 75% to 80% water, whereas older adults are 45% to 55% water. Women tend to have less body water than men due to the effects of hormones and higher amount of adipose tissue, which contains very little water.
Water that moves through the compartments of the body contains electrolytes. Electrolytes are substances that separate or dissociate into ions (charged particles) in solution, and they are abundant in both ICF and ECF. Ions carry either a positive charge (cation) or a negative charge (anion). Major cations and anions are listed in Table 12.2. Electrolyte balance is essential for normal physiologic functioning and is closely linked with fluid balance. In addition, most electrolytes interact with hydrogen ions to maintain an acid-base balance. Electrolyte balance must be kept within a narrow range to maintain homeostasis. Many illnesses and body system alterations cause electrolyte imbalances. Electrolytes are important because they carry electrical impulses to other cells in the body.
The major electrolytes in body fluids are sodium, potassium, calcium, magnesium, chloride, phosphorus, sulfate, hydrogen, and bicarbonate ions and many proteins. Electrolyte concentrations differ in ICF and ECF. Table 12.3 lists the major cations and anions found in body fluids.
Administering intravenous (IV) fluids and electrolyte replacements may be thought of as routine nursing care; however, both require as much diligence and critical thinking as administration of any medication. This chapter describes fluid replacement strategies, pharmacologic management of specific electrolyte imbalances, and nursing care of patients with fluid and electrolyte imbalances.
Homeostasis
Homeostasis maintains a constant internal balance within the body despite the effects of a constantly changing external environment. Two principles stand out when considering homeostasis and fluid and electrolyte balance. The first principle is that anions and cations must be balanced within each compartment and remain electrically neutral. The amount of fluid within each compartment remains constant, and compartments work continuously to maintain fluid balance and replace and exchange ions to maintain neutrality. The second principle is that the fluid compartments remain in osmotic equilibrium except for transient changes.
TABLE 12.1
| Fluid Compartment | Percentage |
| Intracellular fluid | 40% |
| Extracellular fluid | 20% |
| Interstitial fluid | 15% |
| Intravascular fluid | 5% |
| Transcellular fluid | Approximately 1-2 L total but generally not included in calculations |
| Total body fluid | 60% |
TABLE 12.2
| Cations | Anions |
| Potassium (K+) | Chloride (Clˉ) |
| Sodium (Na+) | Bicarbonate (HCO3ˉ) |
| Calcium (Ca2+) | Phosphate (PO4ˉ) |
| Magnesium (Mg2+) | Sulfate (SO4ˉ) |
TABLE 12.3
Electrolyte Concentration in Body Fluids
| Intracellular Fluid | Extracellular Fluid |
| Major Cations | Major Cations |
Potassium Magnesium Sodium | Sodium Potassium Calcium Magnesium |
| Major Anions | Major Anions |
| Phosphorus | Chloride Phosphorus |
Movement of fluid and particles between and within compartments is controlled by a number of processes, including osmosis, the movement of water across a semipermeable membrane from areas of low solute concentration to those of high solute concentration; diffusion, movement of molecules from an area of high concentration to one of low concentration; hydrostatic pressure, the force within a fluid compartment; osmolality, which describes the concentration of fluids; and active transport, which requires metabolic activity and expenditure of energy to move a substance across a cell membrane. The number of solutes in a solution is expressed as a unit of measurement called the osmole, which is particularly useful when referencing osmotic solutions.
Osmolality
Osmolality refers to the number of particles dissolved in the serum, primarily sodium, urea (blood urea nitrogen [BUN]), and glucose. It also is a measure of the concentration of solutes per kilogram in urine. Normal serum osmolality ranges from 275 to 295 mOsm/kg. As the number of particles increases, the concentration of the solution also increases. The change in the concentration of particles will affect chemical behavior and movement of water. Sodium is the primary electrolyte in the ECF and keeps water in this compartment.
The following three types of fluid concentration are based on the osmolality of body fluids:
1. Isoosmolar fluid has the same weight proportion of particles (e.g., sodium, glucose) and water.
2. Hypoosmolar fluid contains fewer particles than water.
3. Hyperosmolar fluid contains more particles than water. The plasma/serum osmolality (concentration of circulating body fluids) can be calculated if the serum sodium level is known or the sodium, glucose, and BUN levels are known.
Hypoosmolality of body fluid may be the result of excess water intake or fluid overload (edema) caused by an inability to excrete excess water. Hyperosmolality of body fluid could be caused by severe diarrhea, increased salt and solute (protein) intake, inadequate water intake, diabetes, ketoacidosis, or sweating.
The terms osmolality and tonicity are similar but not identical. Osmolality refers to the concentration of particles in a solution. Tonicity is used primarily as a measurement of the concentration of IV solutions compared with the osmolality of body fluids.
Fluid Replacement
General Considerations
All routes of fluid intake and loss must be considered when assessing fluid balance. General guidelines can be used as the basis for establishing fluid needs. The recommended water intake for a healthy adult is about 2300 to 2900 mL per day; oral intake accounts for 1200 to 1500 mL, solid foods about 800 to 1100 mL, and oxidative metabolism about 300 mL daily.
Patients lose water daily through various routes: kidneys, skin, lungs, and GI tract. The kidneys, the major organ regulating fluid loss, produce 1200 to 1500 mL of urine daily. Insensible water loss is continuous and occurs daily through the skin and lungs without awareness and is not measurable. Sensible water loss occurs through the lungs/respiration (500 mL/day) and perspiration/skin (500 to 600 mL/day) and through the GI tract/feces (200 mL/day) and is measurable. The minimum urinary output for an adult is 0.5 to 1 mL/kg/h or 35 to 70 mL/h for a 70-kg patient.
Daily water requirements differ according to the patient’s age and medical problems. Intravenous fluids (IVFs) are ordered based on an evaluation of the patient’s fluid and electrolyte balance, fluid requirements, and fluid needs. Several questions must be addressed: Is the purpose of IV therapy replacement or maintenance? What are the patient’s water, electrolyte, and protein requirements? The patient’s weight, caloric needs, and body surface area are other important considerations. Illness and surgery increase the amount of fluids lost and affect fluid and electrolyte needs. Ongoing assessment and monitoring of the patient’s responses to fluid and electrolyte therapy is vital for patients with fluid and electrolyte imbalances.
Intravenous Solutions
With fluid volume deficit (FVD) from the extracellular body compartment, fluid is lost from the interstitial and vascular spaces. Different types and concentrations of IVFs are available to replace body fluid losses.
Types of Intravenous Solutions
The three general classifications of IV solutions used for fluid replacement are crystalloids (with and without added electrolytes), colloids, and blood and blood products.
Crystalloids
Crystalloid solutions contain fluids and electrolytes and are able to freely cross capillary walls. They do not contain any proteins, which are necessary to maintain the colloidal oncotic pressure that prevents water from leaving the intravascular space. Crystalloids are used as short-term maintenance fluids and to treat dehydration and electrolyte imbalances. Crystalloids cause early plasma expansion but have a shorter duration of action than colloid solutions.
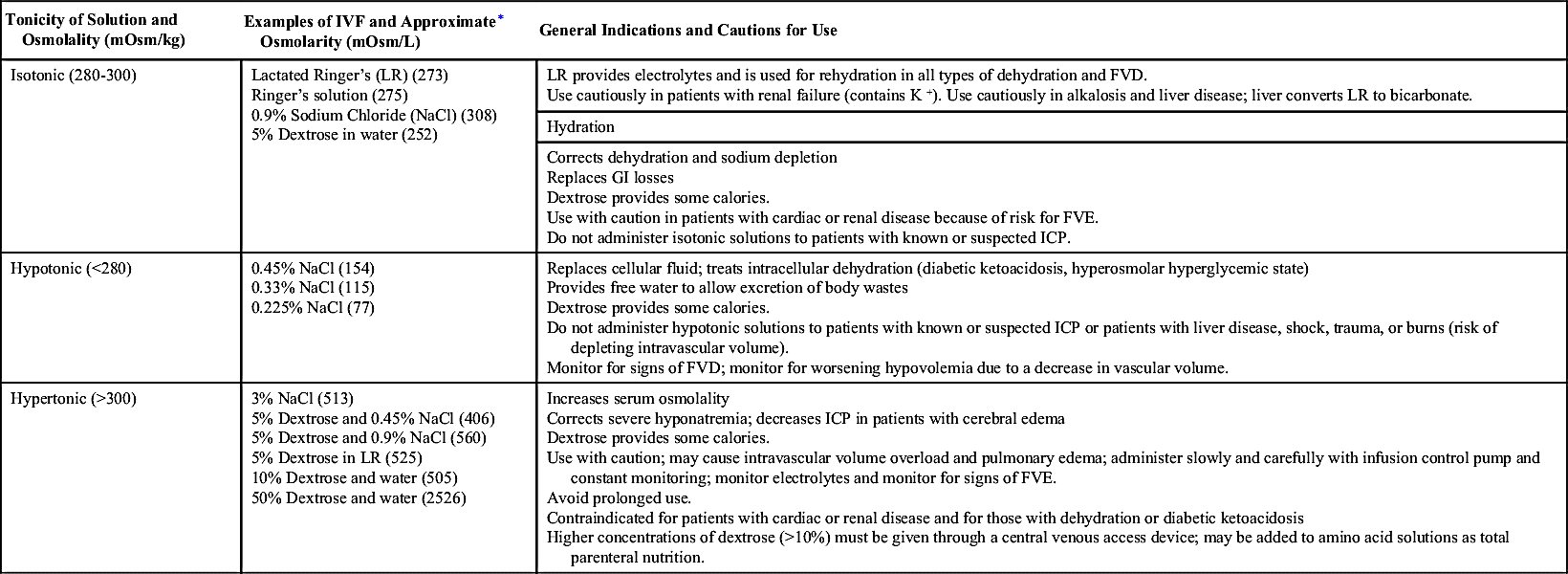
The three major classifications of crystalloid IVFs are isotonic, hypotonic, and hypertonic (Table 12.4):
• Isotonic solutions have the same approximate osmolality as ECF or plasma. Because of the osmotic equilibrium, water does not enter or leave the cell, therefore there is no effect on red blood cells (RBCs). Isotonic solutions are primarily used for hydration and to expand ECF volume, because the fluid remains in the intravascular space.
• Hypotonic solutions exert less osmotic pressure than ECF, which allows water to move into the cell. IV infusions of hypotonic solutions result in an increased solute concentration in the intravascular space, causing fluid to move into the intracellular and interstitial spaces. Excessive infusion of hypotonic solutions may cause hemolysis, decreased blood pressure, and decreased IVF volume.
• Hypertonic solutions exert greater osmotic pressure than ECF, resulting in a higher solute concentration than the serum. When administered, hypertonic IVF fluids pull water from the interstitial space to the ECF via osmosis and cause cell shrinkage. Patients receiving hypertonic solutions must be monitored carefully for signs of circulatory overload (because of the increase in ECF volume).
Parenteral solutions that contain dextrose are available in different concentrations and in combination with other solutions, such as normal saline and lactated Ringer’s solution. Dextrose solutions provide hydration and some calories and increase glucose levels in the blood. The pharmacist will use dextrose solutions to dilute IV medications for administration. The addition of dextrose to an IV solution affects the tonicity of the solution after it is infused. Five-percent dextrose solutions are hypertonic when added to normal saline or lactated Ringer’s solution.
The Infusion Nursing Society’s Practice Guidelines recommend that dextrose solutions higher than 10% should be given via a central vein. An exception is 50% dextrose, which may be given in small amounts via a peripheral vein to correct hypoglycemia. Dextrose can be irritating to veins as a result of the pH of the solution (3.4 to 4). If hypertonic solutions are not diluted and are given peripherally, there is a risk of vein irritation, damage, and thrombosis. Long-term use of hypertonic solutions may result in electrolyte depletion, increased intravascular volume, fluid overload, water intoxication, and pulmonary edema. Rapid infusions of dextrose solutions may cause hyperglycemia, which can lead to osmotic diuresis and fluid and electrolyte imbalance.
Sodium solutions are available in various concentrations and tonicities. Isotonic solutions are primarily used for hydration to expand the ECF and during blood product transfusions. Rapid infusion of isotonic saline solutions may lead to hypernatremia, fluid volume excess (FVE), and electrolyte depletion. Long-term use should also be avoided because normal saline provides no calories. Hypotonic solutions are also used for hydration and to treat hyperosmolar diabetes. Hypertonic saline solutions are used to treat severe hyponatremia or hypochloremia; careful monitoring of electrolyte levels is important to avoid excess replacement.
Balanced electrolyte solutions, such as lactated Ringer’s solution and Ringer’s solution, contain electrolytes (no magnesium) and minimal calories with the addition of dextrose. Their primary use is hydration and electrolyte replacement. However, they do not provide adequate electrolytes for maintenance therapy for patients with limited or no oral intake. Lactated Ringer’s solution is similar to plasma in electrolyte content. Lactate is added as a buffering agent and is metabolized to bicarbonate. Complications of infusions of Ringer’s and lactated Ringer’s include fluid overload, excess electrolytes, and metabolic acidosis with long-term therapy. Because lactate is metabolized in the liver, lactated Ringer’s is contraindicated for patients with liver disease.
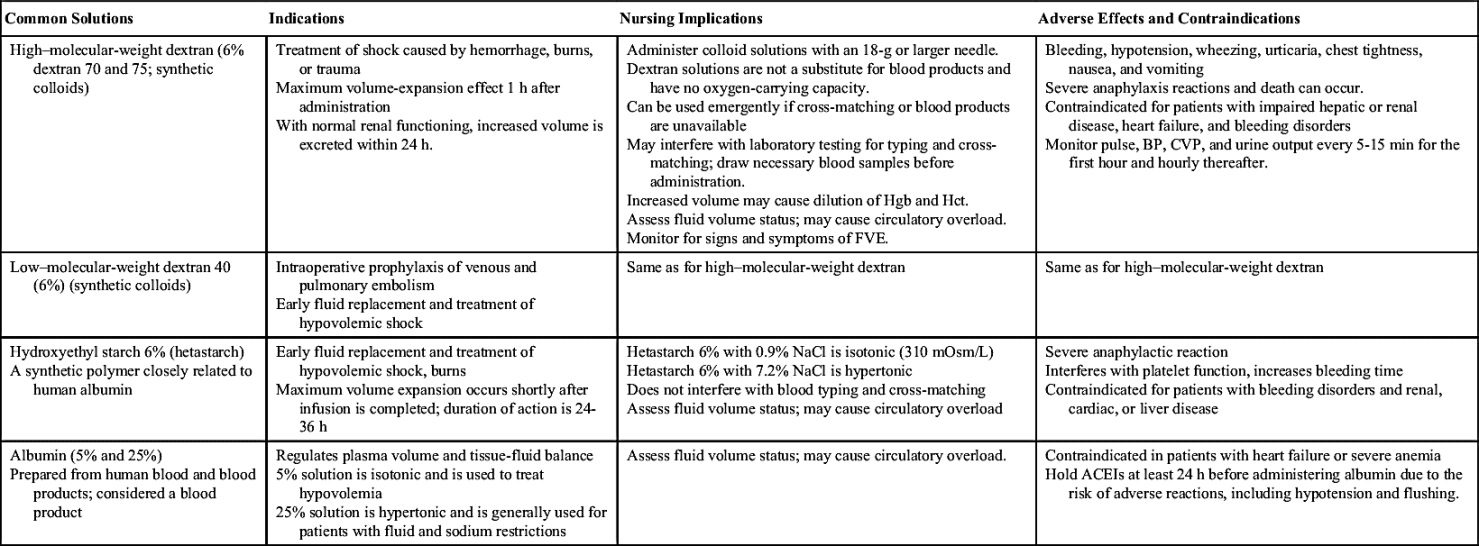
Colloids
Colloid solutions contain protein or other large molecular substances that increase osmolarity without dissolving in the solution. Because of their size, the particles are unable to pass through the semipermeable membranes of the capillary walls and stay within the intravascular compartment; thus colloids are also known as plasma expanders. They act by increasing the colloidal oncotic pressure and pulling fluids from the interstitial space into the plasma, increasing blood volume. The composition of colloid solutions includes proteins, carbohydrates, and lipids. Colloids typically have small and large particles, except albumin, whose particles are all one size. Commonly used colloids are shown in Table 12.5.
Blood and Blood Products
Nurses complete a thorough patient assessment prior to, during, and after administration of blood products. Each facility has a policy and procedure for blood transfusions, and each registered nurse is oriented in the correct procedure. Blood products include packed RBCs (PRBCs), plasma, platelets, and cryoprecipitate. A unit of PRBCs contains concentrated RBCs with most of the plasma and platelets removed; the approximate volume is 350 mL/unit. The approximate volume of a unit of whole blood is 500 mL/unit. Infusing PRBCs over whole blood offers an advantage because packed cells allow an increase in oxygen-carrying capacity with a smaller volume. One unit of whole blood elevates the hemoglobin by approximately 0.5 to 1 g/dL, and one unit of PRBCs elevates the hematocrit by three points.
It is important for nurses to understand the specific blood components prior to beginning the infusion. Proper product-to-patient identification is paramount in preventing transfusion reactions. Reducing errors that increase during blood administration is a focus of the Joint Commission’s National Patient Safety Goals of 2015. Also, the National Healthcare Safety Network’s Hemovigilance Module was developed to increase patient safety and decrease costs associated with transfusion-related adverse events (see www.cdc.gov/nhsn/acute-care-hospital/bio-hemo).
Blood components are administered with a 19-gauge needle, cannula, or catheter. A “Y” tubing set is used with a microaggregate filter with one arm of the “Y” for isotonic saline solution and the other arm for the blood product. The maximum rate of infusion is 4 hours per unit, beginning with removal of the unit from the refrigerator. If the transfusion is not finished by the 4-hour mark, the transfusion bag must be returned to the blood bank, and a new bag of blood is issued to complete the transfusion. Never add medications to the unit of blood.
Loop diuretics are often prescribed to patients receiving whole blood or PRBCs to prevent circulatory overload, particularly in patients who are at risk for developing pulmonary edema and FVE. The patient may also be premedicated with an antihistamine (e.g., diphenhydramine) and hydrocortisone to decrease the possibility of a transfusion reaction. For patients receiving multiple blood transfusions, the serum ionized calcium level should be monitored. Both PRBCs and whole blood products are processed using sodium citrate and citric acid for anticoagulation; multiple blood transfusions can result in a decrease in the plasma calcium levels.
IV fat emulsion, also known as lipid emulsion, is a component of parenteral nutrition for patients who are unable to get nutrition through an oral diet (see Chapter 14). Fat emulsion can supply up to 30% of the patient’s caloric intake and is usually recommended for patients who are unable to tolerate oral or enteral feedings for 7 days or more. The contents of fat emulsion are primarily soybean or safflower and triglycerides with egg phospholipids added as an emulsifier. Parenteral nutrition provides essential nutrients intravenously, including proteins, carbohydrates, electrolytes, trace minerals, and vitamins. Fat emulsion is often added to parenteral nutrition formulas by the pharmacist. The patient must have a centrally or peripherally inserted vascular access device to receive parenteral nutrition formulas. Nausea, vomiting, and elevated temperature have been reported when fat emulsion is infused quickly. It is used cautiously in patients at risk for fat embolism, such as with a fractured femur, and in patients with an allergy to eggs or soybeans and those with pancreatitis, bleeding disorders, liver failure, and respiratory disease.
Electrolytes
Potassium
Potassium (K+) is the major intracellular cation, and 98% of the body’s potassium is found within the cells; only 2% is found in the ECF. Potassium is essential for neuromuscular activity and cellular metabolism, and potassium levels affect cardiac and skeletal muscle activity.
Potassium moves in and out of the cells under the influence of the potassium-sodium pump. It maintains the concentration difference in the cells by pumping potassium into the cells and pumping sodium out. Acid-base balance also influences potassium levels. Acidotic conditions tend to pull potassium out of cells, whereas alkalotic conditions tend to put potassium back into cells. The kidneys are the primary route for potassium loss, eliminating about 90% of the daily potassium intake. An inverse relationship exists between sodium and potassium reabsorption in the kidneys. Aldosterone also plays a role in the excretion of potassium.
Daily dietary intake is necessary because potassium is poorly stored in the body. Recommended daily potassium intake is about 4.7 g (4700 mg), either in potassium-rich foods or as potassium supplements. Foods rich in potassium include tuna and fruits and vegetables such as mangoes, oranges, avocados, tomatoes, cucumbers, spinach, strawberries, and bananas.
Functions
Potassium is necessary for transmission and conduction of nerve impulses and for contraction of skeletal, cardiac, and smooth muscles. It is also necessary for normal kidney function and for the enzyme action used to change carbohydrates to energy (glycolysis) and amino acids to protein. Potassium promotes glycogen storage in hepatic cells, regulates the osmolality of cellular fluids, and plays a role in acid-base balance.
Hypokalemia
Hypokalemia, or potassium deficit, occurs with serum levels below 3.5 mEq/L. Most cases of hypokalemia are caused by excessive loss rather than deficient intake. Whenever cells are damaged from trauma, injury, surgery, or shock, potassium leaks from the cells into the intravascular fluid and is excreted by the kidneys. With cellular loss of potassium, potassium shifts from the blood plasma into the cell to restore the cellular potassium balance; hypokalemia usually results. GI sources of potassium loss include vomiting, diarrhea, suctioning, recent ileostomy formation, draining fistulae, and diuretic therapy. Between 80% and 90% of potassium in the body is excreted in the urine; another 8% is excreted in the feces. Other causes of potassium loss include burns, total parenteral nutrition therapy, alkalosis, prolonged laxative use, excessive licorice consumption, corticosteroid use, increased aldosterone levels, and decreased magnesium levels.
Hypokalemia may not be symptomatic until potassium levels fall below 3.0 mEq/L. It is important to recognize early signs and symptoms to prevent severe hypokalemia, which can result in cardiac arrest and death. Early clinical manifestations include fatigue, muscle weakness, anorexia, nausea, and vomiting. Muscle weakness does not usually occur until potassium levels fall below 2.5 mEq/L. Weakness usually begins in the lower extremities, progresses to the trunk, and may result in paralysis; respiratory muscle involvement may result in respiratory arrest. Quadriceps weakness may be one of the earliest signs of hypokalemia. Severe signs and symptoms include paresthesias, leg cramps, decreased bowel motility and paralytic ileus, confusion, rhabdomyolysis and myoglobinuria, atrial and ventricular dysrhythmias, and cardiac arrest. Electrocardiogram (ECG) changes include flattened or inverted T waves and depressed ST segments. Patients at risk for hypokalemia-induced dysrhythmias include older adults, those with heart disease or coexisting hypomagnesemia, and those on digoxin or other antiarrhythmics. Certain drugs, such as potassium-wasting diuretics and cortisone preparations, promote potassium loss. Patients receiving these drugs should increase their potassium intake by consuming foods rich in potassium or by taking potassium supplements as prescribed by their health care provider. Keep in mind that potassium-sparing diuretics do not deplete potassium stores, so patients would not be requested to increase potassium intake or to take supplements. Serum potassium levels should be monitored periodically for abnormalities.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree