Location
Route of administration
Conjunctiva
External
Cornea
External
Internal eye
Intravitreal
Subconjunctival
External
Intracameral
Eyelids
External
Orbita
Oral
Parenteral
Retrobulbar
Retina
Intravitreal
Oral
Parenteral
Lachrymal apparatus
Oral
Parenteral
External
Eye preparations are also employed for diagnostic purpose or in connexion with surgery. Combinations of fluorescein with oxybuprocaine, proxymethacaine or lidocaine are applied for the measurement of intra-ocular pressure with tonometry, diagnosis of corneal defects and choice of size and control of hard contact lenses. Strips with fluorescein are used to examine the integrity of the tear film. In some countries they are considered medical devices. Sodium hyaluronate eye drops, other eye drops which relieve the symptoms of dry eyes by increasing the viscosity of the tear film or eye preparations in the context of contact lenses are generally regarded as medical devices.
In order to improve bioavailability, active substance targeting and patient compliance new dosage forms with controlled release have been developed: colloidal carriers, implants, inserts, plugs, active substance eluting contact lenses and iontophoresis [3–6].
In community pharmacies contact lens solutions are delivered to customers as medical devices. It is noteworthy to mention that many contact lens wearers do not clean their lenses properly. The careless use of their lenses can result in eye infections. During application of medicated eye preparations contact lenses should not be worn.
In some countries eye preparations are prepared in pharmacies for the special needs of animals. E.g. some breeds of dogs frequently suffer from dry eyes or vascular keratitis. Formulas for veterinary use generally do not differ from those designated for human use.
10.2 Definitions
The description of eye preparations to be used as medicinal products is similar in the European, British, Japanese and US-American Pharmacopoeias. Several categories may be distinguished:
Eye drops
Eye lotions
Powders for eye drops and powders for eye lotions
Semisolid eye preparations (ointments, creams and gels)
Ophthalmic inserts
Eye drops are sterile aqueous or oily solutions, emulsions or suspensions of one or more active substances intended for administration upon the eyeball or instillation into the conjunctival sac.
Eye lotions are sterile aqueous solutions intended for use in rinsing or bathing the eye or for impregnating eye dressings in order to cover the eye.
Semisolid eye preparations are sterile ointments, creams or gels intended for application to the conjunctiva or to the eyelids. They contain one or more active substances dissolved or dispersed in a suitable base. They have a homogeneous appearance.
Ophthalmic inserts are sterile, solid or semisolid preparations of suitable size and shape, designed to be inserted in the conjunctival sac, to produce an ocular effect. They generally consist of a reservoir of active substances embedded in a matrix or bounded by a rate-controlling membrane. The active substance, which is more or less soluble in the lachrymal liquid, is released over a determined period of time. Ophthalmic inserts are individually distributed into sterile containers.
These pharmacopoeial general monographs on eye preparations do not comprise parenteral preparations to be administered in the eye.
10.3 Anatomy and Physiology
Anatomical characteristics and physiological mechanisms protect the eye against toxic external effects. These mechanisms include the specific structure of the cornea, blinking, baseline and reflex lachrymation, drainage, tear film composition and the corneal sensitivity. The combination of all mechanistic, anatomical and physiological characteristics maintains the integrity of the eye, together with immunological and antimicrobial properties of the lachrymal fluid [5, 7–10].
10.3.1 Structure of the Eye
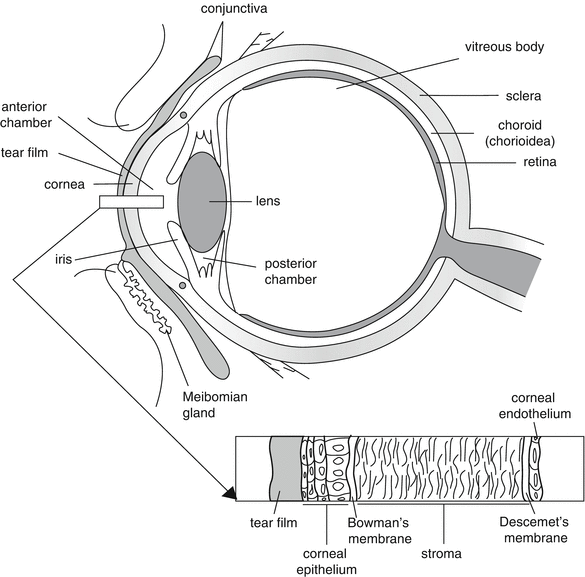
Figure 10.1 shows schematically the structure of the human eye. In detail the structure of the cornea is given. The cornea separates the aqueous humour from the lachrymal fluid and protects the delicate internal structures of the eye from external influences. The cornea is a clear, avascular tissue to which nutrients and oxygen are supplied by the lachrymal fluid and the aqueous humour. It is composed of five layers: a lipophilic multilayered epithelium, Bowman’s membrane, a hydrophilic stroma, Descemet’s membrane and a lipophilic endothelium.
The epithelial cells are closely packed together like a pavement, forming not only an effective barrier to most micro-organisms, but also for active substance absorption. The low permeability of the cornea is due to the presence of tight junctions between the epithelial cells. The superficial corneal epithelial cells are exfoliated from the ocular surface, their average life is 4–8 days.
The cornea is highly innervated with sensory nerves, which serves important sensory and reflex functions.
The eyeball has a wall consisting of three layers: the outer coat or the sclera and cornea, a middle layer or uveal coat and the inner coat or retina.
The cornea has no blood vessels and the sclera only a few, consequently the supply of immunoglobulins to these tissues is limited. Therefore the treatment of infections is difficult.
The conjunctiva is a thin transparent membrane, which lines the inner surface of the eyelids and is reflected onto the globe. The conjunctiva consists of three parts: bulbar on the eye surface (sclera), fornix or conjunctival sac and palpebral on the inner side of the eyelids. The bulbar conjunctiva lies upon the sclera and only attaches to the sclera on the limbus. The structure resembles a palisade and is more permeable than the cornea.
The high corneal sensitivity is due to the specific innervation of the eye. The corneal surface possesses the highest nerve density of all organs in the human body: about circa 7,000 nociceptors per mm2. The nerve endings are located only one layer below the corneal surface. Consequently they are very sensitive and active substances elicit reflex blinking. Three types of stimuli are responsible for pain perception: mechanical, physico-chemical and temperature gradient dependent. The distribution of the various kinds of nerves and receptor is functional heterogeneous: 20 % mechanical, 70 % physico-chemical and 10 % temperature (cold) sensitive. The sensitivity of the cornea and conjunctiva seems to be dependent on the colour of the iris, age and gender [11, 12]. Pathophysiological processes, long term use of ocular medication could influence the corneal sensitivity [13, 14].
The high corneal sensitivity serves to protect the eye. Reducing pain perception is dangerous. Patients should be warned of the danger of anaesthesia dolorosa due to repeatedly instillation of local anaesthetic eye drops. Welding without protection or snow blindness due to excessive exposure to UV light can cause photoelectrical keratitis, which is a very painful condition [15]. Administration of local anaesthetics will be recommended. It is true that local anaesthetics reduce corneal sensitivity but they delay the renewal of corneal epithelium layers with nerve endings. Repeated instillations of anaesthetics result in the duration of action being shortened resulting in pain breakthrough and are responsible for the serious condition where the stroma melts away [16]. Therefore, local anaesthetics should only be delivered in single-dose containers and their use should be limited.
10.3.2 Tear Film and Lachrymal Secretion
The lachrymal glands secrete lachrymal fluid, which spreads on the exposed part of the eye forming the precorneal tear film. An intact film protects the ocular surface from desiccation. The tear film results from the lachrymal functional unit [8] which consists of the:
Lachrymal glands
Ocular surface
Sensory nerves involved
The tear film is a mixture of several excretion products:
Aqueous fluid (95 % of water, salts, glucose, urea, proteins) secreted by the lachrymal glands
Soluble mucins produced by the goblet cells present in the conjunctiva
Lipids from the Meibomian glands embedded in the tarsal plate of the eyelids
The lipid composition is kept within physiological limits by androgens [17]. The decrease of their secretion in elderly people is one of the reasons for development of dry eye syndrome. Patients with Meibomian gland dysfunction show a high tear film evaporation rate and a high tear osmolality [18, 19].
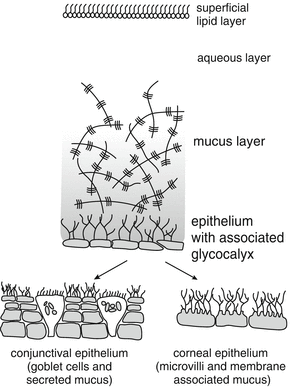
The structure of the tear film
According to the “three layers theory” the tear film consists of a superficial lipid layer, the central aqueous layer and an inner mucus layer.
No clear separation exists between the aqueous layer and the mucus layer because mucins are dissolved in the aqueous layer (see Fig. 10.2).
To maintain the integrity of the tear film is of utmost importance during the administration of eye drops. The role of the glycocalyx is essential. The glycocalyx consists of anionic membrane-spanning or membrane-associated mucins secreted by corneal and conjunctival epithelial cells [20, 21]. Due to its moisture binding characteristics it stabilises the tear film (see Fig. 10.2). Moreover the superficial lipid layer prevents evaporation of the central viscous aqueous layer.
About 1.2 microlitres of lachrymal fluid is secreted per minute. The functions of the lachrymal fluid are:
Improvement or maintenance of the optical quality of vision (homeostasis)
Lubrication of the eyeball
Elimination of foreign bodies
Supply of nutrition to the ocular surface
Defence against infection (viral and bacterial)
Oxygen transport to the avascular corneal epithelium
The secreted lachrymal fluid is spread over the ocular surface by the eyelids (precorneal tear film) and distributed to the conjunctival sac during blinking. Meanwhile the tears are swept to the medial canthus and drained through puncta, canaliculi, lachrymal sac and nasolachrymal duct which opens into the inferior nasal passage. The volume of the precorneal tear film amounts to about 7 microlitres. The conjunctival sac can accommodate about 30 microlitres, but in some persons only 20 microlitres or even less [22]. The tear film evaporates at a rate of 6–12 microlitres per hour [23].
Immunological and Antibacterial Mechanisms of the Eye
The ocular surface is the domain of the mucosa-bonded immune system [24]. This system plays an important role in combating infections by killing micro-organisms. It consists of the lachrymal gland, conjunctiva and related structures. Besides immunoglobulins, enzymes and bactericidal components are present: IgA, lysozyme, lactoferrin, lipocalins, cathelicidine and probably beta-defensins [25]. Lipocalin is considered the most important component in eliminating toxic (phospho)lipids and fatty acids from the ocular surface [26]. Elimination is necessary, otherwise only partial hydration of the corneal epithelium will occur, which could result in ulceration [27].
10.3.2.1 Tear Film Stability
The tear film is only temporarily stable. The time period of stability of an intact tear film is named the tear film break up time (TFBUT) [28]. TFBUT is measured using fluorometry [29]. Tear film stability is reduced by tensioactive preservatives, which solubilise the superficial lipid layer. The example is the preservative benzalkonium chloride. Reduction of stability causes an increase in blinking frequency [30]. The tear film breaks up after 10–20 s and dry spots form on the corneal surface (dewetting of the cornea). These dry spots irritate the corneal nerve endings and activate the lachrymal functional unit, which triggers the blink reflex. During eyelid opening a new protective film spreads over the ocular surface. Patients suffering from dry eye syndrome exhibit formation of dry spots even when eye drops without benzalkonium chloride are instilled. The reduced film stability can be due to lower lipid-, tear- or mucin production [31].
Improvement of the Diagnosis of Dry Eye Syndrome
The Ocular Protection Index (OPI) was proposed to get a better insight into the uncomfortable condition, dry eye syndrome [32]. To obtain an optimal hydration of the corneal epithelium the ratio between the time period of an intact tear film and the time between two reflex blinks must be greater or equal to 1 (OPI ≥ 1). Investigation of tear film stability using sodium fluorescein improves the diagnosis of dry eye syndrome when 1–5 microlitres solution is used instead of larger volumes [33]. As a result the certainty of diagnosis is increased. However, the use of small drop volumes has not yet been introduced, as this technique is insufficiently developed.
Improvement of Tear Film Stability
Patients suffering from dry eye syndrome complain about tear film instability. Stability can be improved by increasing the viscosity of the tear film (see also Sect. 10.4.4) [34]. Viscoelastic polymers increase viscosity but also possess elastic properties. During blinking sodium hyaluronate (Na-HA) exhibits a kind of cushioning action and induces improved protection of the ocular surface compared to classical pseudoplastic viscosity enhancing polymers. Consequently the movement of the eyelids during blinking is smoother. Na-HA is effective in providing relief to dry eyes.
Other viscosity enhancing polymers are natural anionic polysaccharides such as gellan gum (E-418) and xanthan gum (E-415). These macromolecules are used as gelling agents. Artificial tears may contain dextran, hypromellose and carbomer sometimes with or without polyvinylalcohol and povidone. Unfortunately the ideal non crust forming and stability improving gelling agent has not yet been developed. It seems that hydroxypropyl-guar possess more specific adhesive properties to injured ocular surfaces [35–37].
10.4 Biopharmaceutics
After administration active substances should reach their target tissue. Therefore eye drops should fulfil certain requirements. The following properties are important:
The lipophilicity of the active pharmaceutical ingredient (active substance)
Active substance concentration
Dilution by the lachrymal fluid and drainage
Viscosity of the tear film
pH value and buffer capacity of the preparation
Osmotic value of the preparation
First the absorption of the active substances through the cornea is discussed and afterwards how the previously mentioned factors will influence the absorption.
10.4.1 Lipophilicity and Ionisation of Active Substance
As shown in Fig. 10.2 the cornea consists of various layers. The permeation of the active substances occurs transcellular or paracellular. Lipophilic, non-ionised molecules will diffuse via the transcellular pathway, while ionised, hydrophilic molecules pass through the paracellular space (tight junctions). The pore size at the corneal surface is about 6 nm (60 Å). The lipophilic epithelium prevents the passage of 90 % of the hydrophilic active substance dose, but only 10 % in the case of a lipophilic active substance [38]. Most ophthalmic active substances are salts of weak bases, which are completely dissociated at a low pH value. The contrary is true in the case of most NSAIDs. The permeation of pilocarpine [39] and some mydriatics [40] is higher when the molecule is not dissociated, resulting in a higher therapeutic effect compared to the protonated molecule (see Fig. 10.3).
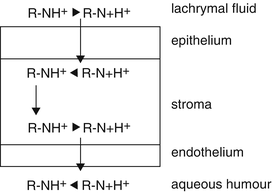

Fig. 10.3
Transport of dissociated active substances through the cornea. Source: Recepteerkunde 2009, ©KNMP
The degree of lipophilicity and ionisation of the active substance determines the extent of corneal permeation. Many examples are reported in the literature [41, 42].
However, recent research proved the presence of a number of transporters in the cornea and conjunctiva such as amino acid/peptide, nucleoside, organic anionic and organic cationic. These systems will influence the absorption of active molecules. Moreover active substance efflux pumps at the cell surface could restrict active substance penetration into ocular tissues [5].
10.4.2 Active Substance Concentration, Drop Size, Surface Tension
The amount of active substance applied to the eye depends not only on the concentration but also on the drop size, which is influenced by the surface tension of the solution. The design, dimensions of the dropper tip, the cross-sectional surface area on which the drop is formed and the dispensing angle at which the patient manipulates the bottle influence the drop volume instilled [43].
Drop size Variation
Research performed in the eighties in the USA showed that most dropper containers delivered drop volumes between 25 and 75 microlitres [44]. A similar study performed about 10 years later demonstrated that drop volume of products on the market had decreased [45]. This trend will continue as production techniques of dropper tips became more precise.
Active substances such as antazoline and tetracaine reduce the surface tension. Eye drops with a surface tension below 35 mN/m (normal surface tension of lachrymal fluid is 40–46 mN/m) are painful and uncomfortable [30].
10.4.3 Dilution and Drainage
The maximum volume of solution the lower conjunctival sac can accommodate is about 30 microlitres. After instillation the normal volume of the precorneal tear film (7–10 microlitres) is established again due to drainage of the extra volume of fluid present. The drainage rate is directly proportional to the volume of ophthalmic solution instilled. A high percentage of hydrophilic active substances are eliminated and lost to the eye. The drained active substance reaches, via the nasolachrymal duct, the nasal mucosae and after absorption enters the systemic circulation. As lipophilic substances are absorbed much more rapidly, these systemic effects are less prominent.
If the decision is taken to administer very small volumes (1–10 microlitres) of concentrated active substance solutions in order to compensate for dilution in the tear film, irritation becomes a problem. Consequently excessive lachrymation, drainage and wash out will occur, resulting in low active substance availability. To avoid irritation the surface tension, osmolality, pH and buffer capacity should be within certain limits (see Sects. 10.4.5 and 10.4.6).
Tolerance and availability of ophthalmic preparations are closely related [46]. The condition being treated can also play a role: i.e. the drainage rate in Sjögren’s patients is slower than in healthy people [47].
Administration of more than one eye drop makes no sense. The second drop or a drop of double volume will be drained almost immediately. The availability could only be improved by lengthen the residence time of the preparation in the lower conjunctival sac. Closing the puncta by applying pressure with the thumb or fingers, but also closing the eyelids for approximately three minutes, increases the residence time and decreases the drainage of the ophthalmic solution to the nasal mucosae [48].
Animal experiments evaluating drop size and percentage of drained active substance demonstrated that after instillation of a 50 microlitres solution more than 50 % was lost [49]. When three drops of flurbiprofen (0.3 mg/mL) were instilled in rabbits, one drop every 30 min, a 2–3 times higher concentration was measured only in the corneal tissues [50].
10.4.4 Viscosity of the Tear Film
To improve the therapeutic effect of a medicine, one should try to increase the absorption of the active substance. Eye ointments and eye creams stay much longer in the conjunctival sac and on the ocular surface than eye drops. Consequently the active substance is delivered during a longer period of time to the eye. Ointments or creams could be considered as prolonged release dosage forms (depot preparations) for ophthalmic use. Viscous eye drops can increase residence time in the eye but are generally less successful. The use of viscous ophthalmic gels is under discussion. Xerogel forming polymers such as cellulose derivatives are in theory able to block for example puncta on the eyelids and canaliculi when used in high concentration and after desiccation. Carbomer, which does not form xerogels, used in the concentration 2 mg/mL, improves the availability and the prolonged activity of some active substances. No blurred vision was reported [51].
10.4.5 pH Value and Buffer Capacity of the Solution
The pH value of the lachrymal fluid is about 7.4. Due to evaporation of CO2 from the tear film when the eyes are open, the pH value increases to 8 and even higher values [52]. Three buffer systems are present in tear fluid: bicarbonate-carbonate, mono-dibasic phosphate and amphoteric proteins; the buffer capacity is low [53]. The acid-neutralising capacity of the tear fluid of one eye is equal to about 8–10 microlitres 0.01 M NaOH.
The pH value influences the active substance availability from an ophthalmic preparation in two ways:
A pH value outside the physiological range causes extra lachrymation and reduces the residence time of the active substance on the ocular surface.
The pH value influences the permeation of the active substance through the cornea (see Sect. 10.4.1).
Solutions with pH values below 5.0 and above 8.5 are uncomfortable and not well tolerated [54]. The intensity and the duration of pain sensation after instillation are related to the acidity (pH) and buffer capacity of the eye drop.
10.4.6 Osmotic Value of the Preparation
Eye drops should be in principal iso-osmotic with lachrymal fluid, which means the NaCl concentration 9 mg/mL or approximately 0,9 % (280 mosmol/L). This value corresponds to the tear fluid of patients suffering from conjunctivitis. In healthy persons the osmolarity of the lachrymal fluid equals 290–310 mosmol/L, but varies during the day [55]. Tears of keratoconjunctivitis sicca and Sjögren’s patients show higher values (343 mosmol/L) [56]. Therefore these patients welcome hypotonic eye drops [57].
Almost no pain sensation occurs within the range 0.5–2 % NaCl [58]. Strong hypo-osmotic solutions could damage the corneal epithelium. This should not happen during normal application of eye drops, because one minute after instillation of distilled water the baseline osmolarity of the tear film is restored.
10.5 Adverse Effects and Toxicity
The irritating properties of substances have been investigated using the Draize irritating test on rabbit eyes [59]. This method was used to test many different substances and resulted in serious consequences for the rabbit eye. Nowadays the test is performed according to contemporary acceptable procedures. The redness and its rate of development are an indication of the irritating potential of the substance examined. No satisfactory alternative method is available. Caution should be taken regarding interpretation of the observations collected. For example the frequency of (reflex) blinking influences the results obtained after application of ophthalmic preparations. The frequency differs between rabbit and human being. The rabbit blinks about every 20 min, while humans every 10 s. This difference in blinking frequency is relevant during investigation of viscous solutions.
Alternative in vitro or ex vivo methods have been developed and validated for assessing ocular irritation [60, 61].
Ophthalmic preparations should not contain substances, which could mechanically injure the eye during the blinking of the eyelids (see also Sect. 10.8). The cornea is extremely sensitive to solid particles especially when larger than 50 μm. Particles of 20–25 μm could, depending on their shape, irritate the eye. Due to the induced lachrymation the active substance will be washed away rapidly.
Even if eye drops are applied topically, undesirable systemic side effects could occur after absorption [43, 62]. The effects could be dangerous to life. Administration of scopolamine eye drops in children resulted in a toxic coma [63]. A substantial amount of active substance administered is drained through the nasolachrymal tube, reaches the nasal mucosae and will be absorbed in the systemic circulation. The correct instillation of eye drops reduces the risk of drainage to the nose but this cannot be completely eliminated. The correct methodology for instilling eye drops will be discussed under Sect. 10.9.
A special mention concerns the use of chloramphenicol in ointment and eye drops, see also Sect. 22.2.4. Chloramphenicol is degraded by light. During preparation and storage the degradation product 4-nitrobenzaldehyde is formed by photolysis. This degradation should be avoided because 4-nitrobenzaldehyde is responsible for a non-dose dependent aplastic anaemia, which condition is rare but lethal. This photochemical reaction can also occur on the ocular surface and skin. Therefore it may be better to apply chloramphenicol as eye ointment at night instead of eye drops at daytime. This might be anyway as effective as the general recommendation of 0,5 % eye drops 3 times a day.
10.6 Product Formulation
A reliable source of information concerning this section can be found under “Codex der Augenarzneistoffe und Hilfsstoffe” published in Ophthalmika [66]. The pharmaceutical, physico-chemical and pharmacological properties of many active substances used for the preparation of eye drops are described.
Initially the formulation of eye drops will be discussed followed by eye lotions, eye ointments and eye creams.
10.6.1 Eye Drops
10.6.1.1 Choice of Active Substance
A soluble active substance is preferred when eye drops are being formulated. When the active substance prescribed is not (or not sufficiently) aqueous soluble, a suspension will be prepared.
Active substances employed in suspension eye drops are usually micronised. Polysorbate 80 or 20 may be used for wetting of the powdered active substances.
Hydrocortisone eye drops (see Table 18.12) is an example of a suspension formulation, where micronised raw material is used. Povidone is used mainly as wetting agent for an effective dispersion of the hydrocortisone acetate. This improves the settling behaviour (see also Sect. 18.4.2.2).
Precipitation or opalescence could occur when the concentration of one of the formulation components is near to its limit of solubility or due to an incompatibility between two formulation components. The appropriate choice of excipients can solve these problems. For example, the addition of citrate to an eye lotion containing zinc sulphate prevents precipitation of zinc hydroxide (see Table 10.2).
Zinc sulfate heptahydrate | 0.25 g |
Borax | 0.53 g |
Boric acid | 1.15 g |
Phenylmercuric boratea | 0.0045 g |
Sodium citrate | 0.5 g |
Water, purified | ad 100 mL |
Borax in aqueous solution associates to form a 2:1 complex with chloramphenicol. Therefore, chloramphenicol 0.5 % eye drops could be prepared with the pH value of the solution adjusted to 7 (see Table 10.3).
Chloramphenicol | 0.5 g |
Borax | 0.3 g |
Boric acid | 1.5 g |
Thiomersal | 0.002 g |
Water for injections | 97.7 g |
Total | 100 g |
Frequently the active substance is not or not readily available for pharmacy preparation. Then a sterile licensed pharmaceutical preparation must be used as starting material. Usually powder for solution for injection (i.v.) is used, sometimes solution for injection, powder for bladder irrigation or other products. The active substances comprise the range of antifungals (e.g. fluconazole, voriconazole, amphotericin B), antibiotics (e.g. vancomycin hydrochloride, cefuroxime sodium, tobramycin, bacitracin) or others, e.g. mitomycin. Detailed information is necessary about the overage with respect to the labelled value, the quantity of excipients, the resulting pH and osmolality. Suitability of the reconstituted solution for intravenous injection does not necessarily mean suitability for topical ophthalmic use.
10.6.1.2 Vehicle
Usually eye drops are formulated as an aqueous solution. If an oil is employed medium chain triglycerides [69] are suitable as a vehicle along with refined castor oil, refined peanut oil, refined sesame oil or mixtures of triglycerides (see Table 10.4 and Sect. 23.3.5).
Ciclosporin | 1 g |
Castor oil, refined | 9.9 g |
Triglycerides, medium chain | 89.1 g |
Total | 100 g |
10.6.1.3 pH and Buffer Capacity
The buffer capacity (see Sect. 18.1.1) of the tear film is low. Consequently the buffer capacity of eye drops should be as low as possible. The acid neutralising capacity of tear fluid of one eye is about 8–10 microlitres 0.01 M NaOH (see Sect. 10.4.5). In order to avoid eye irritation the following rule of thumb is used. The volume of 0.01 M NaOH necessary to adjust the pH of the tear film to 7.4 should be less than 25 microlitres 0.01 M NaOH per dose. Sometimes even the equivalent of 10–15 microlitres 0.01M NaOH may be uncomfortable.
The volume of 0.01 M NaOH needed depends on the acidic ingredients, including the buffer. In simple cases knowledge of the pKa value and the molar concentration of the active substance or the buffer substances enables the estimation of the pH of the solution (see Sect. 18.1.1).
Information on the maximum acceptable amount of H+ ions at a pH > 7.4 is not available and of less relevance.
A drop of 1 % pilocarpine HCl solution has a pH value of about 5.5, which after instillation must be adjusted to 7.4 by the lachrymal functional unit. 2 % and 4 % pilocarpine HCl solutions exhibit a pH value of 5.3 and 4.0 respectively, and more NaOH will be required to compensate for the pH difference. If the amount of NaOH needed is higher than the neutralising capacity of the tear fluid, instillation will be painful. The choice of a different salt of the active substance can potentially reduce the irritation caused by ophthalmic preparations. E.g. epinephrine HCl eye drops are less painful compared to epinephrine bitartrate.
For example, pilocarpine HCl, phenylephrine HCl and lidocaine HCl solutions in concentrations higher than 10 mg/mL possess such a high buffer capacity that during administration substantial pain is experienced resulting in lachrymation and wash out of the eye drop. This is due to their high therapeutic concentrations and their pKa values in the neutral or slightly acidic range. Therefore, the pH value of these eye drops should be adjusted as near as possible to 7.4.
The pH value of pilocarpine solutions on the market is 4 and is irritating due to the low buffer capacity of the tear fluid. Therefore the LNA formulated pilocarpine eye drops with a pH value of 6.5, this improves the tolerance of the preparation [71].
When the pH value of the eye solution deviates from 7.4, it will take time to get the normal pH restored in the tear fluid. The greater the buffering capacity is, the longer it will take [72]. Therefore, it is advisable not to use buffering solution outside the pH range 6.5–8.5.
In order to obtain well tolerated eye drops, the pH of the active substance solution is measured and if necessary a combination of excipients is added to adjust to the required value (see Table 10.5).
Table 10.5
Combination of excipients used to adjust the pH value of eye drops
Decreasing pH | Increasing pH |
|---|---|
Boric acid | Borax (Na2B4O7.10H2O) |
Sodium dihydrogen phosphate dihydrate (NaH2PO4.2H2O) | Disodium phosphate dodecahydrate (Na2HPO4.12H2O) |
Citric acid, anhydrous; citric acid, monohydrate | Sodium citrate |
Acetic acid | Sodium acetate trihydrate |
Addition of the excipients mentioned in Table 10.5 increases the osmotic value of the preparation. Due to incompatibility or a high osmotic value of the active substance solution, the substances cannot be always employed. In these cases a diluted HCl or NaOH solution is recommended. The disadvantage is of course that an amount of solution instead of solid powder must be weighed or measured. A pH increase can also be carried out with trometamol (pKa > 8).
Exact buffer compositions and osmotic values are reported in [66].
It is preferable to use a boric acid-borax buffer, because this buffer system has a very low buffer capacity at the pH value of the tear film and at any lower pH. Boric acid is a weak acid. Boric acid-borax buffer solutions reacts neutral to weakly basic.
Boric acid and borax are regarded as reproductive toxicants. The use of boric acid in eye drops for children younger than 3 years old is not recommended, but it is permitted since a clarification in 2003 [73]. Boric ions do not permeate through the intact corneal epithelium [74].
The EMA’s committee for medicinal products for human use (CHMP) considered that the benefits of phosphate-containing eye drops outweigh their risks, but that in very rare cases patients with significant damage to the cornea may develop corneal calcification during treatment with eye drops that contain phosphate [75].
10.6.1.4 Viscosity
The mean viscosity of tear fluid is between 1.3 and 5.9 mPa∙s [76]. As expected, increasing the viscosity of eye drops increases the residence time in the conjunctival sac [77]. Not only the viscosity, but also tensioactive properties, adhesion on the ocular surface and interactions with mucins play a role in increasing residence time.
Viscosity enhancing agents intended for use in eye drops must fulfil several requirements. Their chemical and physical characteristics must be stable during and after sterilisation. Sterilisation induces an important viscosity decrease for some polymers. Moreover viscous polymer solutions should be free of particles, colourless, be optically clear and have a refractive index comparable to tear fluid (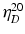 = 1.336–1.338). The concentration used should not cause discomfort and irritation.
= 1.336–1.338). The concentration used should not cause discomfort and irritation.
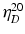 = 1.336–1.338). The concentration used should not cause discomfort and irritation.
= 1.336–1.338). The concentration used should not cause discomfort and irritation.10.6.1.5 Viscosity Enhancing Polymers
In Table 10.6 the characteristics of most frequently used viscosity enhancing polymers are reviewed. More information is available in literature [77] and Sect. 23.7.
Table 10.6
Overview of viscosity enhancing agents used in eye drops preparations (see also 23.7)
Carbomer | |
Hyprolose (hydroxypropylcellulose, HPC) | Hyprolose is used in the production of inserts, such as Lacrisert®. The macromolecules can adhere to the eyelashes, they glue them together |
Hypromellose (hydroxypropylmethylcellulose, HPMC) 4,000 mPa∙s; 0.125–0.5 % or 1.25–5 mg/mL | Hypromellose is a non-ionic cellulose polymer. Hypromellose is a component of the viscous vehicle hypromellose-benzalkonium solution (see Table 10.10). The concentration is % (10 mg/mL), but as it will be diluted 1:1 during preparation of viscous eye drops, the final concentration will be 0.5 % (5 mg/mL). Hypromellose solutions are not always well tolerated because of surface tension reduction of the tear film [30]. The antimicrobial activity of benzalkonium chloride is only slightly influenced by hypromellose [82] |
Methylcellulose (MC) 4,000 mPa∙s; 0.5–1.25 % or 5–12.5 mg/mL | Methylcellulose is a non-ionic cellulose polymer. The high viscosity types of methylcellulose are employed, because at low concentration solutions are viscous enough and the refractive index is only slightly changed |
As with all cellulose ethers, methylcellulose increases the residence time of the preparation. In addition, methylcellulose possesses wound healing properties. Therefore the polymer is suitable as a tear substitute for dry eye especially for those with punctate lesions. A disadvantage are irritating insoluble cellulose particles present in methylcellulose. The amount of insoluble particles depends on the quality of the product | |
Carmellose (carboxymethylcellulose sodium, Na CMC) | The adhesion of carmellose to the ocular mucosa is less than carbomer. The solubility of the polymer depends on the degree of substitution. The viscosity decreases during heating and at a pH value lower than 5 |
Poly(vinyl alcohol) (PVA) 1.4 % or 14 mg/mL | The viscosity and surface tension depend on the degree of polymerisation of the PVA selected. PVA is often a component of artificial tears and contact lens solutions. PVA solutions (viscosity = 25 mPa∙s) sometimes irritate, because of its inherent surface active properties [30] |
Povidone (polyvidone, PVP) K 30 | Povidone is used in the preparation of suspensions in order to facilitate resuspendability of the sediment on shaking. Complex formation between PVP and methyl parahydroxybenzoate or propyl parahydroxybenzoate is possible |
Apart from the polymers mentioned in Table 10.6 some authorised medicines contain other viscosity enhancing agents such as dextran, hydroxyethylcellulose, hyaluronic acid and hydroxypropylguar gum (HP-guar; Systane®) [36, 37, 57]. Hyaluronic acid possesses good adhesive properties. In situ-gelling systems, such as gellan gum, are used in order to increase the precorneal residence time of the eye drop and to obtain a sustained active substance release [78, 79].
10.6.1.6 Preservatives
During the development of an eye preparation whose formulation contains an antimicrobial preservative, the necessity for and the efficacy of the chosen preservative must be demonstrated. The effectiveness of the preservative in the final preparation is tested according to the Ph. Eur. Efficacy of antimicrobial preservation (see Sect. 32.8).
Testing of Antimicrobial Activity
The methodology used to test the antimicrobial activity is still under debate.
A high storage temperature could reduce the antimicrobial activity as seen during the use of a new contact lens solution ReNu with moistureLoc® formulated with a new preservative alexidine [85]. The use of this commercial product caused a Fusarium keratitis epidemic worldwide. Research at room temperature and at high temperature (60 °C) has shown, contrary to other preservatives, that alexidine loses its antimicrobial activity at higher temperature. The cold supply chain of the product is of primary importance. The possible contribution to the development of the biofilm on the contact lens surface was also investigated, but was not considered to have contributed to the problem [86]. The researchers concluded that temperature control during production, storage and transport is of utmost importance. Examination of possible biofilm formation was relevant, because other studies investigated this phenomenon as possible origin of infections. In general, attention is drawn during antimicrobial efficacy tests to planktonic free moving bacteria contrary to microorganisms fixed in biofilm structures. Nowadays interest in biofilm formation (see also Sect. 19.3.5) has increased, because bacteria associated with such systems are more difficult to kill [87].
If eye drops do not contain antimicrobial preservatives (Tables 10.7 and 10.8) they are supplied in single-dose containers or in multidose bottles preventing microbial contamination of the content after opening.
Tetracaine hydrochloride | 1 g |
Borax | 0.01 g |
Sodium chloride | 0.7 g |
Water for injections | ad 100 g |
With thiomersal | Without a preservative | |
|---|---|---|
Indometacin | 0.1 g | 0.1 g |
Borax | 0.3 | 0.3 g |
Disodium phosphate dodecahydrate | 3 g | 3 g |
Sodium dihydrogen phosphate dihydrate | 0.25 g | 0.25 g |
Mannitol | 1.6 g | 1.6 g |
Thiomersal | 0.002 g | – |
Water for injections | 94.75 g | 94.75 g |
Total | 100 g | 100 g |
Antimicrobial preservatives should be omitted in eye drops intended for use in surgical procedures. Tetracaine hydrochloride eye drops (Table 10.7) comply with the Ph. Eur. efficacy of antimicrobial preservation.
The use of preservatives is not possible when the patient is sensitive or allergic to the preservative or if eye drops will be administered just before, during or after surgery, because of its toxicity. Commercial ophthalmic products without preservatives are popular because of their better tolerance and lower irritancy potential [88–90].
The preference is given to the combination of benzalkonium chloride and sodium edetate (EDTA). Edetate is added in order to improve the activity of benzalkonium chloride against Pseudomonas aeruginosa.
Benzalkonium Chloride / Edetate and Active Substance Effect
Could the combination of benzalkonium chloride and edetate present in so many ophthalmic solutions influence the therapeutic activity of the active substance? In the case of an intact cornea a higher active substance availability is assumed because benzalkonium chloride acts as a penetration and solubility enhancer increasing passive diffusion of the active substances through the corneal epithelial cells (transcellular pathway). Additionally, edetate is a penetration enhancer, active at the tight junctions between cells, and has an effect on intercellular passive diffusion. Research performed using ketorolac eye drops on rabbits with intact and de-epithelialized corneas [91] demonstrated that the availability of ketorolac in the case of intact corneal epithelium was similar after application of drops with or without benzalkonium chloride and edetate, whilst in the case of the injured cornea a lower availability was measured in the presence of benzalkonium chloride. The researchers speculate that the non-irritating ketorolac formed an irritating combination with benzalkonium chloride resulting in lachrymation (active substance wash out) and lower availability. Combination of edetate with boric acid and an experimental active substance in an ophthalmic solution seems to exhibit permeation increasing properties ex vivo on intact rabbit cornea [92]. The results of both studies do not provide sufficient information to draw a meaningful conclusion as to whether benzalkonium chloride and edetate influence the availability of ophthalmic medicines.
When the combination of benzalkonium chloride and edetate cannot be used because of incompatibilities, thiomersal sodium can be used. Phenylmercuric borate is not available anymore because of toxicological problems to the environment.
A third preservative is chlorhexidine in the form of chlorhexidine acetate or chlorhexidine digluconate at a concentration of 0.1 mg/mL. However, chlorhexidine induces many chemical incompatibilities (see Sect. 23.8).
The preservative selected reduces the choice of other excipients required to adjust pH and osmotic values. Table 10.9 shows the possible combinations of preservatives, pH modifiers and excipients that can add up to the right osmotic value. Table 10.10 shows how these possibilities have led to standard basic solutions (vehicles) for eye drops and concentrates for further dilution.
Table 10.9




Possible combinations of excipients for basic ophthalmic solutions
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree