Fig. 15.1
Necessities of extemporaneously dispensed drug formulations for ocular therapeutics
Considering the eye as an unique organ, understanding of its structure is very essential to develop ophthalmic dosage forms. Medications for ocular diseases have been developed as a special category of drug formulations, namely, ophthalmic products. It requires specific considerations during formulation optimization regarding sterility, stability, compatibility, tonicity, and other physicochemical characteristics relevant to sympathetic understanding of structure of the eye. In spite of commercially available branded ophthalmic drug products, the standard manner of compounding the drug products is another choice through the services of compounding pharmacists (Yuen et al. 2002).
Some examples of compounded substances used in ophthalmology are bevacizumab prepared for intravitreal injection, mitomycin C for surface neoplasms, fortified antibiotic drops used to treat corneal ulcers, and autologous serum or serum tears for the treatment of dry eye (Daniels 2010).
Although pharmaceutical industry has been involved in enhancing the armamentarium of ophthalmic preparations, extemporaneous compounding is often necessary for the successful treatment of specific ocular emergency cases (Giam et al. 2012). In recent years the uses of extemporaneously compounded ophthalmic drug formulations have remarkably increased. It represents the growing knowledge of extemporaneously compounded ophthalmic drug formulations and attempts to standardize these products by suggesting strengths, routes of administration, appropriate vehicles, and methods for their preparation (Buurma et al. 2003). It is still now solely based on the experience and proficiency of compounding pharmacists on how to make them available in a ready-to-use form for patients. Ophthalmic preparations compounded in the way purely based on experience present a risk factor regarding effective assessment of quality and efficacy of the finally compounded product. It is an utmost important duty of pharmacists to ensure the compounded product will be of desired quality and stable for its indicated shelf life (Spark 2014).
According to the leading professional organizations for ophthalmology professionals (Tortora and Grabowski 2002), it is also imperative to have certain ophthalmic drugs handy in the ambulatory setting. Of course, ophthalmologists are not the only clinicians who use medications from compounding pharmacies. For years, many other medical practices, such as ambulatory surgery, orthopedic surgeries, pain management, pediatrics, etc., have relied on compounding pharmacies to supply frequently needed sterile drugs. Whenever the drug has been prescribed according to the best knowledge of physicians, it seems to be an evidence-based practice. When a drug formulation is prescribed to a patient, it is therapy, whereas, when a new drug or in a novel manner of administration, a particular drug formulation at specific strength/dosage is prescribed by clinicians, it is research. Extemporaneous preparations are generally being compounded to meet specific purposes in clinical trials.
In certain ocular emergencies, waiting or delay in treatment while waiting for extemporaneously prepared formulations might get into adverse consequence as shown in the Table 15.1.
Table 15.1
Outcomes of delayed treatment in ophthalmology practice
Drug | Recommended indications | Result of delayed treatment |
|---|---|---|
Intravitreal antibiotics | Endophthalmitis | Permanent loss of vision and even loss of the eye itself |
Fortified topical antibiotics | Bacterial corneal ulcers | Corneal perforation, corneal scarring, or partial or complete blindness within a short period |
Intravitreal vascular endothelial growth factor (VEGF) inhibitors (e.g., bevacizumab) | Neovascularization, neovascular glaucoma (immediate and aggressive treatment is imperative) | Complete loss of vision |
Mitomycin C | Treatment of failing glaucoma filtration procedures and intraoperative treatment at the time of glaucoma filtration surgery | Fibrosis, scarring, and blindness |
Combination dilating drops | Diagnostic use in pediatric patients | Difficulty in receiving multiple eye drops and their related toxicity (especially pediatric patients) |
One size cannot fill the requirement of particular medication for all patients; this is true in medicine as well (Spark 2014). Pharmaceutical industry produces medicines having a limited range of doses and dosage forms which meet the needs of most of the people, but there are some people who require drug/dosage forms that are not manufactured. When licensed manufactured pharmaceutical products do not meet a person’s medicine requirements, then products prepared specifically for them in a pharmacy (extemporaneous preparations) can only fill the inevitability (IACP 2014; Williams et al. 2013).
The intention of this chapter is to explore the implications of extemporaneously prepared ophthalmic drug formulations in the treatment of several ocular diseases. It intends to provide a detail insight into the basic principle involved in extemporaneous ophthalmic preparations, its need, typical issues encountered in the compounding, and benefits in current therapeutics relevant to specific ocular emergency cases. This chapter focuses on different aspects, and physicochemical factors associated with its compounding, evaluation of its quality, safety, efficacy, and other critical parameters and stringent conditions required for its manufacturing in hospitals have been discussed. Future prospects of extemporaneous drug formulations have been discussed for better advancement of pharmacy practice toward the patient care.
15.2 Extemporaneous Ophthalmic Drug Formulations
The word “extemporaneous” is derived from the Latin extempore which is referring to in accordance with the needs of the moment. Extemporaneous dispensing is the science of medicinal preparations as a result of there being no commercially available medicinal product or required form of a medicinal product for the treatment of a patient. In other words, compounding can be referred as patient-specific production of medicinal substances rather than commercially manufactured drug products (Houck 2005).
“Extemporaneous compounding is defined as the preparation, mixing, assembling, packaging, and labeling of a medicinal product based on a prescription order from a licensed practitioner for the individual patient.” The lack of commercially available formulations for patients with specific needs poses a challenge to gain access to the medicinal product in distinct form. This qualifies as off-license use of a medicine, whereby a licensed medicine is reformulated into a preparation that is made acceptable for the needs of the patient (Shargel 1997; Aquilina 2013; USP convention 2011).
“Good Manufacturing Practices” are the guidelines that pharmacists and manufacturers must follow to guarantee that a product is extemporaneously compounded appropriately (NABP 1993). Getting an approval for drugs for ocular specific personalized indication is a very difficult task, due to this extemporaneous preparations help in providing available medications as per need of individual by doing preparation of sterile formulations in aseptic conditions.
15.3 Benefits and Its Utility in Ophthalmology
Many of the medications commonly used in ophthalmology practice are either not available commercially as required by prescribers or do not have FDA approval for particular indication to be used in ocular case which explains why a required ophthalmic drug might not be manufactured as given below (Stokowski 2013; McElhiney 2013):
1.
The product might not be stable enough or have a long enough shelf life to make it feasible to produce on a commercial scale. Some compounded antibiotic ophthalmic preparations (topicals and intraocular injections) are prepared from commercially available injections, but the stability of the drug in the solution or vehicle may only be 14 days or less when refrigerated.
2.
Patients don’t always tolerate the commercial products, usually due to the preservatives in case of pediatric ophthalmology and may need a compounded preparation, for example, benzalkonium chloride.
3.
Some dyes are not commercially available, such as brilliant blue G, and compounders who specialize in high-risk compounded sterile preparations are needed to meet these needs of the ophthalmologists.
4.
Concentration matters in case of ophthalmic products as per physician. A few commercially available antibiotic ophthalmic products are not concentrated or strong enough to treat a severe infection, so the pharmacist may have to prepare a fortified antibiotic ophthalmic solution that has a higher dose of drug and can be effective against a severe infection. For example, sodium ascorbate 10 % w/v is needed to treat corneal alkali burn.
5.
Economics (for the drug manufacturer) may be a factor. It simply may not be profitable for a manufacturer to produce the ophthalmic on a commercial scale or for every class of patients.
15.3.1 Categories of Ophthalmic Products That Must Be Compounded (Stokowski 2013)
1.
Formulations or combinations of drugs that are not commercially available. Examples are combination antimicrobial drops and combination anesthetic and dilating agents.
2.
Patient-specific formulations that must be compounded individually. Examples are autologous serum eye drops that are compounded using the patient’s own serum. Moreover formulations might also require the removal of ingredients that are not tolerated by the patient such as preservatives and antioxidants/bisulfites.
3.
Discontinued yet still needed drugs. Drug makers might have discontinued the production of a drug because the market for the product is too small and it is no longer economically feasible to produce.
4.
Repackaging of doses of available drugs. When the available dosage form is not appropriate for routine clinical need, drugs are fractionated and relabeled for use by clinicians (e.g., bevacizumab).
5.
Manufacturing shortages. When critical drugs are in short supply at the point of manufacture, drugs can be compounded from bulk ingredients.
6.
Ophthalmology practitioners have turned to compounded agents for economic reason. In the case of a drug frequently compounded for ophthalmic use – bevacizumab – cost entered into the equation.
15.3.2 Common Examples of Extemporaneously Dispensed Drugs
Various categories of drugs for ocular use are being dispensed in the hospital pharmacies which include several topical and intravitreal formulations given in Table 15.2.
S. No. | Drug | Dosage strength | Indications |
|---|---|---|---|
1. | Bevacizumab | 1.25 mg/0.05 mL | Wet Age-related macular degeneration |
2. | Ganciclovir | 2.0 mg/0.1 mL | CMV retinitis in HIV patients, varicella-zoster retinitis, acute retinal necrosis |
3. | Foscarnet | 2.4 mg/0.1 mL | |
4. | Vancomycin | 1 mg/0.1 mL | Gram-positive bacterial endophthalmitis |
5. | Ceftazidime | 2.25 mg/0.1 mL | Gram-negative bacterial endophthalmitis |
6. | Amikacin | 0.4 mg/0.1 mL | |
7. | Amphotericin B | 0.1 mL of 5–10 μg/mL | Fungal Endophthalmitis |
8. | Dexamethasone | 0.4 mg/0.1 mL | Bacterial endophthalmitis |
9. | Triamcinolone acetonide | 0.1 mL of 4 mg/mL | Diabetic Macular edema, central retinal and branch retinal vein occlusions, wet age-related macular degeneration |
15.3.3 Cost-Effective Dispensing of Bevacizumab (Anti-VEGF Antibody)
Anti-vascular endothelial growth factors (anti-VEGF) have revolutionized the treatment of many retinal diseases. The VEGF inhibitor bevacizumab (AVASTIN, Genentech, Inc.) was approved by the FDA for the treatment of colorectal cancer in 2004. Bevacizumab is a full-length, humanized monoclonal antibody directed against all the biologically active isoforms of vascular endothelial growth factor (VEGFA). Its potential for the treatment of ocular pathologies, such as choroidal neovascularization, age-related macular degeneration (AMD), diabetic retinopathy (DR), and retinal vein occlusion, has been well recognized by the ophthalmologists (Salvatore and Focke 2007).
Although found to be safe and effective in clinical ophthalmology practice, the ophthalmic use of bevacizumab comes under the off-label use, and therefore not manufactured in doses required for intravitreal injection (Wong and Kyle 2006). Subsequent to bevazicumab, ranibizumab (another anti VEGF antibody) was approved for ocular use by FDA (Biswas et al. 2011). Both of the agents were subsequently found to be equally clinically effective in treating AMD, but a huge difference between them is the cost. The per-dose cost of Avastin is $30–$50, but for Lucentis, it is $2000. The reason for the discrepancy is that a 4-mL single-dose vial of Avastin can be divided by a compounding pharmacy into as many as 10–18 aliquots (depending on the techniques used) for individual injection, whereas Lucentis is supplied in the volume needed for a single injection for ophthalmic use (Schmucker et al. 2012; Velpandian et al. 2007).
The price difference was largely responsible for the decision by many ophthalmology practices to obtain their supplies of bevacizumab for intravitreal injection from national compounding pharmacies, rather than using single-dose vials of ranibizumab. A recent study provided support for this decision, finding that given their similarities in efficacy, bevacizumab is more cost-effective than ranibizumab (Rogers et al. 2013; Velpandian et al. 2007). Compounding pharmacies prepare batches of bevacizumab in single-dose syringes/ampoules, in accordance with the prescription and make the aliquot available to ophthalmology specialists directly. This allows ophthalmologists to have the sterile, dispensed drug in hand when patients need unscheduled intravitreal injections and is essential for the provision of prompt, efficient, and effective patient care.
15.4 Comparison of FDA-Approved Branded/Generics and Compounded Drug Products
As such extemporaneously compounded drug products are not under any regulatory bodies like FDA, but at the same time, its quality is solely governed by the skill of compounding pharmacists, principles of pharmacy practice, and facilities available. State Board of Pharmacy is the only regulatory body oversees pharmacy practice including drug compounding. In the countries where there is no specific regulatory requirements regarding sterile compounding requirements, they must voluntarily adopt the requirements set by other regulatory agencies like USFDA for maintaining their standards and patient’s safety. By necessity, compounded drugs are made under standards that are less stringent than those applied to FDA-approved products but, without any compromise on sterility and without adding any other active pharmaceutical ingredient (API). The branded products are FDA approved for particular indication as well as they are under strict regulations for any adverse events reported. With the development of industrial manufacturing, the pharmacies transitioned into dispensaries which are under oversight and regulation. Therefore, extemporaneously prepared ophthalmic product must be prepared in a highly recommended facility and guidelines documented by FDA/USP for sterile compounding (Glass and Haywood 2006). Generally, extemporaneous formulations lack studies to document stability, bioavailability, pharmacokinetics, pharmacodynamics, and safety (Nahata and Allen 2008) unlike branded as well as generic drug products are systematically evaluated. Extemporaneously prepared drug formulations of already marketed drug products are exempted from reporting any adverse event to the FDA which is mandatory for FDA-approved products.
Following are the brief details given (Table 15.3) of key differences between FDA-approved (branded and generics) and compounded drug products.
FDA-approved drug | Compounded drug | |
|---|---|---|
Made “extemporaneously” after receipt of prescription | No | Yes |
Reviewed by FDA for quality, safety, and efficacy prior to marketing/prescribing | Yes | No |
Manufactured under federal GMP regulations | Yes | No |
Labeling for safe prescribing and use required and regulated | Yes | No |
Sterile products adhere to federal GMP sterility requirements | Yes | No |
Benefit-risk assessment | Conducted by FDA at population level | Conducted by prescriber at patient level |
15.4.1 Regulations in the USA and Other Countries
Government regulations must be implemented on all compounding pharmacies for their better functions and control standardization. Compounding pharmacies and pharmacists should be regulated by their individual State Board of Pharmacy, FDA, and the Drug Enforcement Agency (DEA) (Ann 2013; IACP 2012). The United States Pharmacopeia (USP <797>) sets the national standards for the process, testing, and verification of any medication prepared for administration to patients. Pharmacies with the Pharmacy Compounding Accreditation Board (PCAB) accreditation status have demonstrated that they meet the highest possible standards and are recognized as having another level of quality assurance for both sterile and non-sterile compounded preparations (Cabaleiro 2007, 2008; Murry 2008). Although, the guidelines are very clear in USFDA, other drug regulatory agencies in various countries are having their own regulatory methods and distribution of powers among various enforcement authorities on compounding pharmacies.
15.5 Ophthalmic Compounding/Dispensing
Extemporaneous compounding of ophthalmic drugs needs to be done with strict compliance of USFDA (USP <797> guidelines 2012). The ophthalmic preparations are sterile preparations according to the USP <797> standards and must be prepared in the sound sterile environment risks to patient health and safety are higher if sterile agents are improperly compounded. Drugs meant for ophthalmic treatment must ensure its sterility, otherwise contaminated ophthalmic medication with bacteria, fungi or particulate matters can further worsen the patient’s ocular condition.
In ophthalmic compounding and dispensing, the formula (components) for the compounded medication is very important. Components that might be safe for oral or parenteral use could be toxic or irritating to the eye. To make the administration of the ophthalmic preparation comfortable for the patient, the pH and tonicity must be adjusted. Ophthalmic products should be prepared by taking into account the perfect balance between formulation characteristics (pH, osmolarity, preservation) and environmental factors (clean room, aseptic handling, and trained pharmacy professionals). The following factors given in Fig. 15.2 must be taken into account prior to make/dispense ophthalmic formulations extemporaneously.
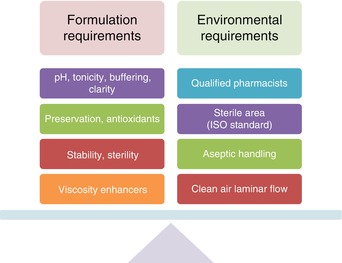

Fig. 15.2
Illustrate common requirements of ophthalmic preparations
15.5.1 Dispensing Laboratory (Pharmacy): Minimum Requirements of the Hospital Pharmacy for Adopting Extemporaneous Dispensing
A suitable dispensing facility in the pharmacy would be having sterile room fitted with a centralized HEPA/ULPA filters or suitable filters to get clean air. These clean rooms maintain particulate-free air by using aforesaid filters employing laminar or turbulent airflow principles. The construction of these rooms must be done according to the laid down conditions of the state drug regulatory bodies like USFDA or EU. As ocular drugs are recognized as solutions meant for ocular use are sterile in nature, this unit must accompany a moist heat, dry heat, ethylene oxide (ETO) sterilizing instruments. The water used for the formulations used in pharmacy must meet the standards of water for injection according to the pharmacopeia. Generally, freshly distilled water is used for the formulations in dispensing pharmacy, and the use of deionized high purity water prepared by reverse osmosis systems is still a matter of debate for its substitution in the place of distilled water unless it reaches required quality in terms of chemical, microbiological, and pyrogen test of water for Injection according to the pharmacopeia (USFDA 2015 & CHSP 1996).
15.5.2 Components of Ophthalmic Formulations
Ophthalmic solutions are sterile solutions that are compounded and packed for installation into the eyes. It provides more uniform dosage forms. In addition to their sterility, their preparation requires the careful consideration of pharmaceutical factors as the need for antimicrobial agents, isotonicity, buffering, viscosity, and proper packaging. Safe, sterile compounded medications have long been essential tools available to ophthalmologists for urgent treatment of eye diseases and conditions, benefitting patients (Parke 2013). These products are compounded in the most sterile and safest manner possible in ensuring the safe availability of these medications for eye physicians and surgeons and the patients they serve (Nahata and Allen 2008).
15.5.3 Desired Qualities of Ophthalmic Solutions
The properties of an ideal ophthalmic solution are described below. These are not just desired properties but absolute requirements for ophthalmic solutions. Achieving all of these objectives simultaneously is sometimes impossible, and therefore appropriate compromises can be made, or another therapeutic option may be necessary (Dale et al. 2013; Sandle 2014):
A.
Sterility and clarity: The preparation and packaging should be done in a sterile environment such as a laminar flow hood or a barrier isolator. A sterile, particle-free solution can be achieved by one of the following methods:
1.
The solution should be prepared in a manner similar to a parenteral preparation, using aseptic technique with sterile parenteral drug products as the solution ingredients and packaging the solution in a clean, particle-free, sterile container.
2.
It should be prepared by using non-sterile but high-quality ingredients and filtered using a 0.22- or 0.45-micron bacterial filter/0.1-micron mycoplasma-free filter into a dispensing container that is clean, particle-free, and sterile.
3.
If an autoclave is available, terminal steam sterilization can be used. In this case, the solution may be prepared using non-sterile but high-quality ingredients and packaged in an appropriate clean, particle-free container that is stable to the elevated temperature and pressure needed for steam sterilization. The preparation is then autoclaved in the dispensing container. Quality control procedures for steam sterilization must be used, for example, validation of the autoclave cycle through the use of biological and other indicators, as well as the use of monitoring devices that track and record time, temperature, and pressure. Since steam sterilization uses elevated temperature and pressure, consideration of drug stability is important before using this method.
All ophthalmic solutions should be clear and free from any particulate matter. Foreign particles in an ophthalmic solution can cause damage to the eye by causing abrasions to the cornea and the membranes of the eyelids or other possible diseases such as endophthalmitis in case of intravitreal injections. Filtering the solutions with a 0.22-micron filter should remove all harmful particulate matter. The use of HPMC can improve the clarity of ophthalmic solutions. Adding Polysorbate 20 and Polysorbate 80, in a maximum concentration of 1 %, can also improve the clarity of ophthalmic solutions. Polysorbates, also known as polyoxyethylene sorbitan fatty acid esters, are solubilizing agents that help dissolve poorly soluble ingredients.
B.
Preservation: When the solution is dispensed in a multidose container that is to be used over a period of time longer than 24 h or more, a preservative must be added to ensure microbiologic safety over the period of use. It is necessary to add preservative in the ophthalmic solutions to prevent microbiological contamination during use in ocular diseases. Usually preservative-free dispensed ophthalmic solutions should be used at earliest.
C.
pH: Although solutions with the same pH as lacrimal fluid (7.4) are ideal, the outer surfaces of the eye tolerate a larger range, 3.5–8.5. The normal useful range to prevent corneal damage is 6.5–8.5. The final pH of the solution is often a compromise, because many ophthalmic drugs have limited solubility and stability at the desired pH of 7.4. Buffers or pH-adjusting agents or vehicles can be added to adjust and stabilize the pH at a desired level. Ophthalmic solutions are ordinarily buffered at the pH of maximum stability of the drug(s) they contain. The buffers are included to minimize any change in pH during the storage life of the drug; this can result from absorbed carbon dioxide from the air or from hydroxyl ions from a glass container. Changes in pH can affect the solubility and stability of drugs; consequently, it is important to minimize fluctuations in pH. The buffer system should be designed sufficient to maintain the pH throughout the expected shelf life of the product, but with a low buffer capacity so that when the ophthalmic solution is instilled into the eye, the buffer system of the tears will rapidly bring the pH of the solution back to that of the tears. Low concentrations of buffer salts are used to prepare buffers of low buffer capacity.
D.
Isotonicity: Solutions that are isotonic with tears are preferred. An amount equivalent to 0.9 % NaCl is ideal for comfort and should be used when possible. The eye can tolerate tonicities within the equivalent range of 0.6–2 % NaCl without discomfort. There are times when hypertonic ophthalmic solutions are necessary therapeutically or when the addition of an auxiliary agent required for reasons of stability supersedes the need for isotonicity. A hypotonic ophthalmic solution will require the addition of a substance (tonicity adjusting agent) to attain the proper tonicity range.
E.
Stability: The stability of the ophthalmic formulation determines the therapeutic efficacy of drug product. As with all pharmaceutical solutions, ophthalmics must be chemically, physically, and microbiologically stable. It is the most intensive parameter toward the quality of ophthalmics. After every preparation, pharmacists should incorporate the beyond-use date on the compounded product.
F.
Therapeutic efficacy: This is the only criteria which depends on the overall stability of active ingredient(s) in the product. The active ingredient(s) should be present in the most therapeutically effective form. This goal must often be compromised for reasons of solubility or stability of the active ingredient or patient comfort. For example, while many drugs are most active in their undissociated form, they are least soluble in this form. They may also be less stable at pH values that favor the undissociated form.
G.
Compatibility with the eye: Most of the ingredients in ophthalmic solutions should be incorporated with prior knowledge of toxicity to the eye. The products should be free of chemicals or agents that cause allergy or toxicity to the sensitive membranes and tissues of the eye. Auxiliary agents, such as preservatives and antioxidants, should be added with care because many patients are sensitive to these substances. Before adding any auxiliary agent, it is recommended to check the history of patient about allergies and sensitivities.
Sourcing Bulk Drugs and Quality Control
In the art of compounding, the most important aspect is the process to ensure the quality of the ingredients for compounding pharmacy. Unlike mass commercial production, in dispensing pharmacies, the bulk drug container needs to be opened multiple times for making small batches. Therefore, it is advised that the bulk drug must be immediately packed into multiple small airtight containers. Moreover, it is wise to check the bulk drug powders; it must be tested for infrared spectroscopy for its appropriateness at least once or twice in a year as per the standard operating procedure. The source of such drugs must be ensured that they comply with pharmacopoeial requirement and its material data sheet along with analysis.
15.5.4 Active Ingredients
The active ingredients used in ophthalmic liquids are available as pure powder, as sterile powder manufactured for parenteral administration, or as a sterile, parenteral solution of the desired ingredient. It is worth mentioning here that most of the ocular drugs are available as their corresponding salts. Therefore, one must remember to calculate their equivalent weight to the active drug component before making the formulation under extemporaneous prepared solutions. For example, homatropine HBr with the MW 356.25 g/mol must be calculated for its equivalence to homatropine having MW 275.34 g/mol unless otherwise specified.
15.5.5 Auxiliary Agents
Auxiliary agents added to ophthalmic solutions include buffers, tonicity adjustors, preservatives, antioxidants, and viscosity-inducing agents (Table 15.4).
Category | Examples |
|---|---|
Buffers | Sorenson’s phosphate buffer |
Citrate buffer | |
Acetate buffer | |
Boric acid buffer | |
Tonicity adjustors | Sodium chloride, sodium nitrate, sodium sulfate, dextrose, glycerol, propylene glycol, mannitol |
Preservatives | Benzalkonium chloride (0.004–0.02 %) |
Benzethonium chloride (0.002–0.01 %) | |
Chlorobutanol (0.5 %) | |
Phenylmercuric acetate (0.001–0.01 %) | |
Phenylmercuric nitrate (0.001–0.01 %) | |
Thimerosal (0.005–0.02 %) | |
Parahydroxybenzoates: | |
Methyl paraben (0.1–0.2 %) | |
Propyl paraben (0.02–0.04 %) | |
Antioxidants | Sodium bisulfite (0.01–0.5 %) |
Sodium metabisulfite (0.01–0.5 %) | |
Thiourea (0.002–0.3 %) | |
Disodium edetate (0.005–0.1 %) | |
Viscosity modifiers | Polyvinyl alcohol (0.1–4 %) |
Polyvinylpyrrolidone (0.1–2 %) | |
Methylcellulose (0.2–2.5 %) | |
Hydroxypropyl methylcellulose (0.2–2.5 %) | |
Hydroxyethyl cellulose (0.2–2.5 %) | |
Hydroxypropyl cellulose (0.2–2.5 %) | |
Dextran 70 (0.1–3 %) | |
Polyethylene glycol 400 (0.2–1 %) |
15.5.6 Sterilization Procedures in Compounding Pharmacy
All ocular formulations are considered as sterile and have been treated like the way it has been treated for parenterals. Terminal sterilization is an ultimate parameter which makes most of the large volume and small volume parenterals to be sterile. However, for the drugs terminal sterilization can not be given for antibodies like bevacizumab maintaining aseptic zone right from the beginning of the initiation of process for dispensing.
Most of the cases, when vials are taken into compounding room, the main culprit for contamination comes from the carton boxes used for transportation. In order to maintain storing temperature, they are usually dispensed along with ice packs. Therefore, as soon as they are opened, they need to be surface cleaned with disinfectors like spirit or other suitable evaporable surface-sterilizing agents. Once the seal is broken, rubber seal needs to be surface sterilized with disinfectants. It is essential to leave for enough time to give right exposure time for the agents to sterilize the surface. In agents like umbilical cord serum or autologous serum or any other compounds having biological nature, it is wise to handle them aseptically and to use 0.22-micron sterile in-line filters before placing them in dispensing vials.
15.5.7 Packaging and Labeling of Ophthalmic Products
Dropping vials meant for topical eye drops must have been made up of nontoxic high low-density polyethylene resin or polypropylene resin container having dropping nozzles and screwable cap with piercing tip. All the parts of the vials are usually packed appropriately and sterilized by either 60Co gamma irradiation or by ethylene oxide gas sterilization using required protocols. These sterilizations must be accompanied by the use of indicator tapes to ensure the achievement of complete sterilization process. For its appropriate usage in laminar flow benches, they must be packed in small quantities and sealed in transparent polyethylene bags so that they can be used appropriately whenever there is a requirement of small quantity.
The pharmacist should dispense sterile ophthalmic products into multidose sterile container for packaging and labeling. If the patient is sensitive to the preservative, it is possible to exempt by using preservative-free solution. FDA regulations for sterile ophthalmic products allow the use of unpreserved multidose packaging if it is packaged and well labeled to provide adequate efficacy and minimize the microbial contamination (21CFR 200.50).
15.5.8 Environmental Quality Monitoring
The final quality embedded in sterile ophthalmic formulations is the result of several attempts starting from quality status of the components incorporated, the process utilized, personnel performance, to the environmental conditions under which the dispensing is performed. The principal goal of environmental control is to achieve and maintain sterility and overall freedom from contamination (USP <797> 2012). Aseptic dispensing is an art for which well-qualified personnel (pharmacists) are required serving as an essential component in the pharmaceutical industry, hospital facility, and at clinical settings.
There are a number of guidelines available (Table 15.5) in relation to aseptic dispensing, the usual method of preparation of ophthalmic dosage forms. Aseptic processing is highly regulated, and there is considerable guidance in the US Code of Federal Regulations (CFR 21, such as CFR 21 Subpart C (211.42)), FDA documents, and in the EU GMP “Rules and Guidance for Pharmaceutical Manufacturers and Distributors” (Euradlex 2014).
Table 15.5
Sources of guidelines for aseptic processing
S. no. | Guidelines | References |
|---|---|---|
1. | FDA Guidance for Industry 2004 on Drug Products Produced by Aseptic Processing | USFDA (2004) |
2. | USP <1116> Microbiological Control and Monitoring of Aseptic Processing Environments | USP 35-NF30 <1116> (2012) |
3. | ISO 13408 Aseptic Processing of Healthcare Products | ISO 13408–1 (1997) |
4. | ISO 14698–1. Cleanrooms and Associated Controlled Environments–Biocontamination Control: Part 1: General Principles and Methods | ISO 14698–1 (2003) |
5. | ISPE Baseline Guide to Sterile Manufacturing Facilities | ISPE (1999) |
15.6 Facility Design, Clean Rooms, and Aseptic Handling
Sterile pharmaceutical dispensing for ophthalmic formulations must be attempted in the facility specifically fabricated to have controlled atmosphere which is free from particulate matter microbial contamination (Sandle 2014). This clean room is expected to have separate zones where particulate matter and microbial load are controlled to specific limits according to the classification of clean roams in ISO standards (Table 15.6).
Table 15.6
Classification of Cleanrooms (USP <797> 2004) (International Organization of Standardization (ISO) Classification of Particulate Matter in Clean Room Air (limits are in particles 0.5 μm and larger per cubic meter (current ISO) and cubic feet (former Federal Standard No. 209E, FS209E)))
Class name | Particle Number (maximum limit) | ||
|---|---|---|---|
ISO class | U.S. FS 209E class | ISO, m3 | FS 209E, ft.3 |
3 | Class 1 | 35.2 | 1 |
4 | Class 10 | 352 | 10 |
5 | Class 100 | 3520 | 100 |
6 | Class 1000 | 35,200 | 1000 |
7 | Class 10,000 | 352,000 | 10,000 |
8 | Class 100,000 | 3,520,000 | 100,000 |
In these facilities, air filtration and temperature control are achieved by dedicated systems. For filtering air, high-efficiency particulate air filters (HEPA) are used to control air dynamics. HEPA filters are known to have the efficiency to remove particulate matter which is having more than 0.3 μm in size thereby having the efficiency to remove 99.97 % and has the probability of having particles having the size lesser than 0.3 μm contributing to 0.03 %. An ISO Class 5 environment (approximately equivalent to EU and WHO GMP Grade A or Class 100) is required for aseptic filling/dispensing of ophthalmic products (Table 15.6). To reach these requirements, temperature-controlled HEPA-filtered airflow is designed to dilute and remove airborne particles. Airflow adjustments according to floor design can lead to an improvement with better particle counts (Sundstrom et al. 2009).
As per the USP guidelines, sterile product preparation facilities utilize laminar airflow workbenches to provide an adequate critical site environment. This is either an enclosed barrier unidirectional airflow device or within an isolator. All sterile compounding in a community pharmacy/hospital pharmacy should be performed in clean room inside the laminar airflow hood. These hoods are designed to reduce the risk of airborne contamination during the preparation of sterile products. Laminar airflow hoods have two basic functions, namely, (1) to filter bacteria and exogenous materials from the air and (2) to maintain constant airflow out of the hood to prevent contaminated room air from entering the hood. Airflow velocity determines the filtering capacity of the hood. If airflow is reduced, the filter is presumed to be clogged with contaminants and must be cleaned. The airflow velocity of 0.3 m/s and 0.45 m/s for vertical and horizontal laminar hood, respectively, is recommended (USP <797> 2004). The clean room and laminar flow hood must be tested on regular basis for bacteria and endotoxin.
Engineering controls reduce the potential for airborne contamination in workspaces by limiting the amount and size of contaminants in the processing environment. Primary engineering controls are used and generally include horizontal flow clean benches, vertical flow clean benches, biological safety cabinets, and barrier isolators. Primary environmental control must provide at least ISO Class 5 quality of air to which sterile ingredients and components are directly exposed.
In aseptic dispensing, the process of preparation of ophthalmic products involves the careful handling of sterile materials in a controlled environment to control microbial and particulate contamination to acceptable levels. Careful considerations should be given to aseptic operations. These include the class of clean rooms, areas where the product is transferred into the aseptic processing area and where and how the product is to be dispensed. Regular monitoring of clean room, sterility, and contamination control is necessary to achieve the quality products through aseptic processing (Figs. 15.3 and 15.4).
 < div class='tao-gold-member'>
< div class='tao-gold-member'>





Only gold members can continue reading. Log In or Register to continue
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


