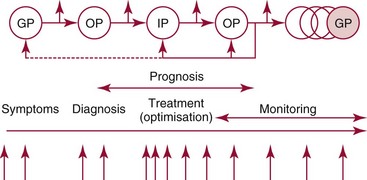
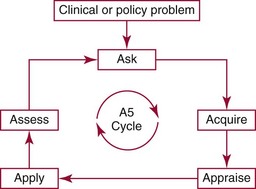
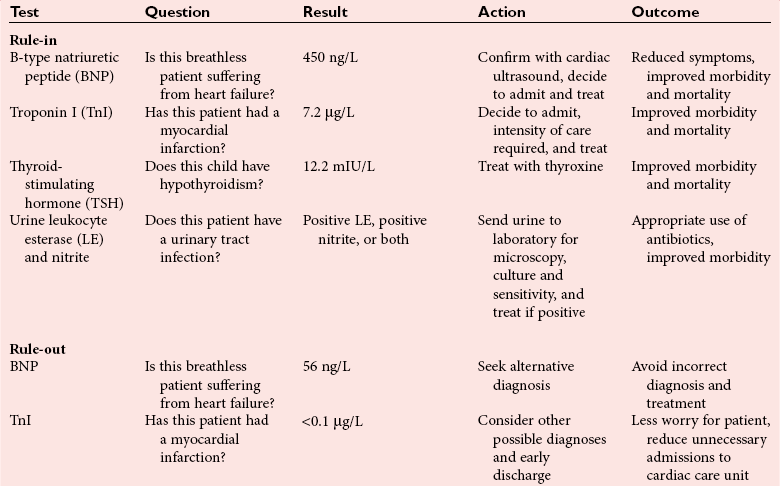
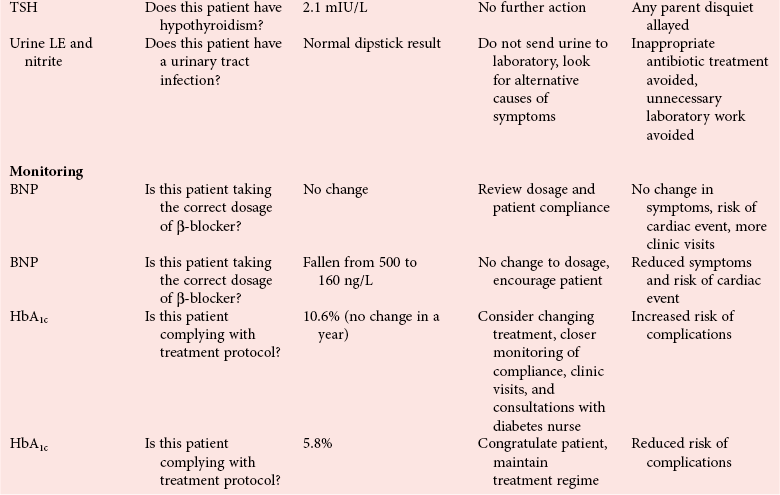
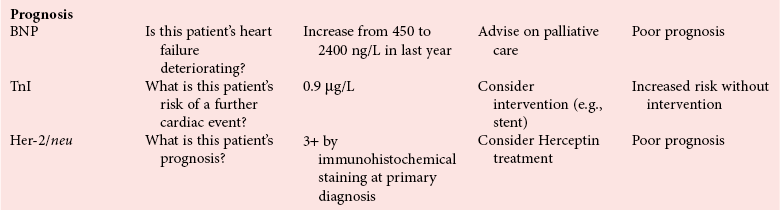
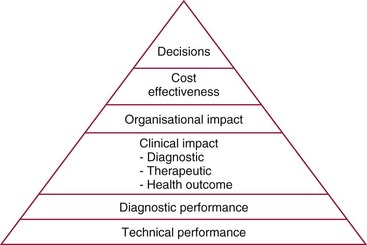
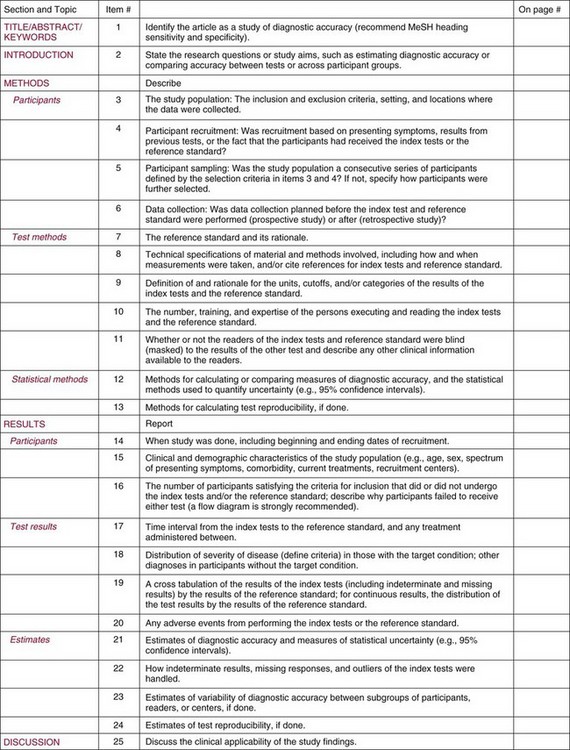
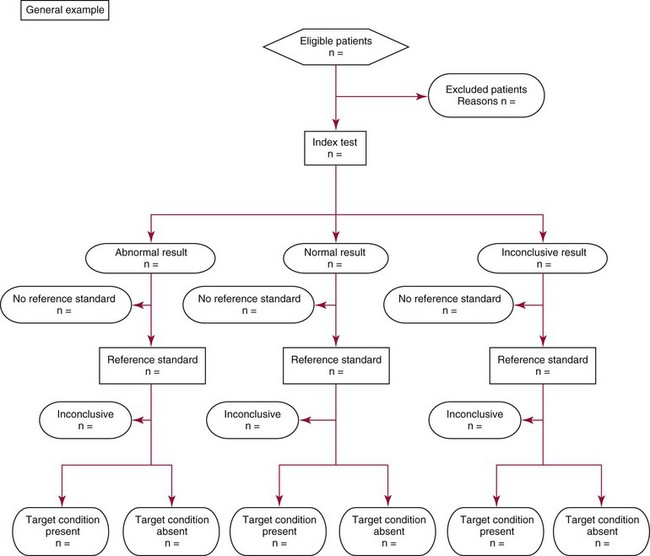
Chapter 4 Christopher P. Price, Ph.D., F.R.S.C., F.R.C.Path., Patrick M.M. Bossuyt, Ph.D. and David E. Bruns, M.D. As described in Chapter 1, the term laboratory medicine refers to the discipline involved in the selection, provision, and interpretation of diagnostic testing, together with the associated decision making, which uses primarily samples of biological fluids provided by patients. The field of laboratory medicine comprises a number of disciplines, including clinical chemistry (also known as clinical biochemistry), hematology, blood banking (transfusion medicine), clinical immunology, microbiology, and virology. Within this structure there may be a range of subdisciplines, such as toxicology and drug monitoring, endocrinology, hemostasis, genetics, parasitology, and mycology. In some parts of the world, laboratory medicine also encompasses cytology and anatomic pathology (histopathology). However, from the perspective of the patient and the clinical user, these are artificial delineations, as they see the laboratory as a single key diagnostic resource that plays a critical role in clinical decision making. Molecular techniques have come into routine use in recent years, often being developed from an initial academic interest in one particular part of laboratory medicine (e.g., biochemical genetics, identity testing, bacterial subtyping). However, these techniques are being used more widely across the spectrum of laboratory medicine; thus although the analytical element of the service may be consolidated, the emphasis now is on the integration of information to address the clinician’s or the patient’s needs. Indeed, with the advent of more complex automation, integrating a wider range of analytical methods onto a single platform, aligned with high throughput capability, the boundaries between disciplines are becoming blurred (e.g., with the advent of “blood sciences” laboratories). This notwithstanding, the analytical components of these specialties are delivered from central laboratories or through a more distributed type of service involving smaller satellite laboratories and point-of-care testing (POCT), or both. Although the core of the practice of laboratory medicine is based on the preanalytical, analytical, and postanalytical elements of routine service, it is underpinned by research and education (with respect to users of the service, as well as those delivering the service), with the whole delivered efficiently through strong clinical leadership and business management. In many cases, the laboratory medicine service also encompasses clinical service, involving direct patient care. The preanalytical service is concerned with ensuring that the right patient gets the right test at the right time, while the analytical service is concerned with getting the right result. The postanalytical service works to ensure the right interpretation of the result, that the right decision is made, and that the right action is taken—with the overall objective of obtaining the best outcome for the patient. Quality management of the whole process is an important feature of both leadership and management of the service, including service accreditation and clinical governance embracing data management, quality control and proficiency testing, clinical audit, and benchmarking. Some of the concepts of good laboratory practice are discussed in greater detail in other chapters, including automation (see Chapter 19), quality control and quality management (see Chapter 8), and the theory and practice of reference values (see Chapter 5), as well as the broader concept of the use of diagnostic tests (which are illustrated in several of the ensuing chapters). However, it is worth pointing out at this early stage that the term diagnostic test is a bit of a misnomer, as the test result is not always used to make a diagnosis; this will be discussed in greater detail when we discuss the underlying concepts of EBM and EBLM. The use of the laboratory medicine service will vary according to the perspective of the customer, or user. Thus the patient may be interested in one specific question, as might the clinician at the time that he/she sees the patient. They and others may take a longer term view, looking at the way the service can help in longer term management of the patient. The purchaser or commissioner of the service, as well as a policymaker, may take a more holistic view, embracing the full care pathway, or patient journey—a journey in which the laboratory may play different important roles at different stages. Furthermore, different stakeholders may have different expectations.98 It can be helpful in understanding this to consider the care pathway and the points at which testing may be relevant in a hypothetical pathway, as illustrated in Figure 4-1. Thus testing in laboratory medicine may be directed at (1) screening an asymptomatic individual for early evidence of the presence of disease, (2) confirming a clinical suspicion (which could include making a diagnosis), (3) excluding a diagnosis, (4) assisting in the selection and optimization of treatment, (5) monitoring compliance with a treatment protocol, and (6) providing a prognosis. Within this framework, test results can be used to establish and monitor the severity of a pathologic disturbance. EBM has been defined as “the conscientious, judicious, and explicit use of the best evidence in making decisions about the care of individual patients.”121 It has been described in terms of “the integration of best research evidence with clinical expertise and patient values.”51 A key objective of EBM is “to incorporate the best evidence from clinical research into clinical decisions.”51 The words and phrases of these succinct definitions and descriptions of EBM warrant further examination and thought, as they reflect many of the reasons for the development of these concepts, as well as some of the challenges we face in laboratory medicine. EBM has also been described as “trying to improve the quality of the information on which decisions are based” and in terms of “thinking not about mechanisms but about outcomes.”44 This comment indicates that the importance of EBM lies not in focusing on the science of the condition, or the pathology of the disease, but on how the intervention (test or treatment) can improve the health outcome. The first comment by Glasziou speaks44 of “the quality of the information” akin to the “best evidence,” referred to in the definition by Sackett and associates,121 both groups emphasizing the use of such information in making decisions. This is why the concepts of EBM are applicable to laboratory medicine, as laboratory medicine is one of the fundamental tools used in making decisions about the care of patients in the practice of medicine. The concepts of EBM and EBP were derived from initial discussions among a group of epidemiologists at McMaster University preparing a series of articles advising clinicians on how to read clinical journals. Out of these discussions came the term critical appraisal; later, the idea of bringing “critical appraisal to the bedside” was born. Implicit in this thinking was the need for good quality evidence and the ability to appraise that evidence and to determine whether it was applicable to the problem at hand and the decision(s) to be made. The initial group grew and evolved into the Evidence-Based Medicine Working Group under the leadership of Gordon Guyatt. The term evidence-based medicine first appeared in an editorial by Gordon Guyatt in 1991,48 and the Working Group subsequently went on to produce a portfolio of papers under the title, Users’ Guides to the Medical Literature.51 Many books have now been written on the concepts, teaching, and practice of EBM which illustrate its origins in clinical epidemiology, as well as the challenges faced in adopting this approach to the practice of medicine and to healthcare management and policymaking.44,93,119,138 Today many thousands of papers and many textbooks address the application of EBM and EBP in all branches of medicine. The justifications for an evidence-based approach to medicine are founded on the constant requirement for information,103 the constant addition of new information,92 the poor quality of access to good information,1 the decline in up-to-date knowledge or expertise or both with advancing years of an individual clinician’s practice,24 the limited time available to spend with the patient, let alone read the literature,122 and the variability in individual patients’ values and preferences. To this one might add, particularly in relation to laboratory medicine, (1) the lack of awareness of the clinical questions being asked by clinicians, with a consequent lack of focus on patient benefit, (2) the limited number and poor quality of studies linking test results to patient benefit,85 (3) the poor perception of the value of diagnostic tests, (4) the ever-increasing demand for tests, and (5) the disconnected approach to resource allocation (reimbursement) in laboratory medicine—silo budgeting—which addresses only laboratory costs without consideration of benefit outside the laboratory, thus forcing decisions to save expense in the laboratory with insufficient attention to the needs of patients, their caregivers, and payers. Guyatt and colleagues49 summarized the practice of EBM as follows: “An evidence-based practitioner must understand the patient’s circumstances or predicament; identify knowledge gaps and frame questions to fill those gaps; conduct an efficient literature search; critically appraise the research evidence; and apply that evidence to patient care.” Efficient, and effective, practice of EBM requires the following: • Knowledge of the clinical process and the ability to convert the clinical problem into an answerable question • Facility to generate focused clinical questions • Facility to generate evidence [i.e., information (through primary research or searching of the literature)] • The ability to critically appraise information to generate knowledge • A critically appraised knowledge resource • The ability to use the knowledge resource • A means of accessing and delivering the knowledge resource • The ability to apply the knowledge appropriately to the clinical problem • The ability to integrate the knowledge with previous experience in the context of the problem at hand • A framework of clinical and economic accountability • A framework of quality management, namely, proper clinical and financial governance The identification of a clinical problem is both the starting point and the foundation of the service provided by the healthcare professional. Doctors and other healthcare professionals are constantly asking questions, and these can be divided into background questions and foreground questions. Background questions, to date more commonly observed being asked by newly qualified professionals by virtue of the way they have been taught, typically deal with knowledge (or underlying science) of the condition (e.g., Why is the circulating level of troponin I increased in a patient suffering with an acute coronary syndrome?). Foreground questions are related specifically to the application of knowledge, of experience in treating the condition and using tests (e.g., Will a troponin I measurement help me determine whether this patient is suffering from an acute coronary syndrome?). Clinicians tend to ask more foreground (and fewer background) questions as their experience develops. This may change in coming years as a more evidence and outcomes–based approach to teaching medical students, and training doctors, evolves. Richardson and coworkers115 argued that all clinical problems could be expressed in the form of a question, and went on to describe a framework for formulating an answerable question: the PICO framework. This will be described in detail later, when the practice of EBLM is discussed. In the area of laboratory medicine, as described later in this chapter, the goal can be expressed in terms of answering a clinical question; appropriate laboratory investigations help to answer the question.108,109,112 Knowledge of the characteristics of these investigations is needed to decide which test to use, when to use it, and how to interpret the results. A knowledge resource in the form of a systematic review (see later in this chapter) should provide critically reviewed evidence of the efficacy, benefits, limitations, and risks of using a test, intervention, or device. Access classically has been gained through scientific journals and textbooks; electronic communications of various sorts (including textbooks and journals) are making access faster and more up-to-date. Professional bodies are now beginning to move away from narrative review to the generation of practice guidelines, their synthesis being based on the discipline of critical appraisal and the process of systematic review. Indeed many health purchasing and commissioning organizations are looking to agencies [e.g., the Agency for Health Research and Quality (AHRQ), www.ahrq.gov, accessed on February 12, 2009] and the National Institute for Health and Clinical Excellence (NICE) (www.nice.org.uk accessed on February 12, 2009) to develop practice guidelines through identification of the best evidence available, generated through both primary and secondary research. When a doctor first sees a patient, that doctor will go through a routine of questioning and observing to identify the signs and symptoms that may be associated with the current health problem; from this process, he/she seeks to establish hypotheses about their etiology. Competing hypotheses may need to be resolved; this may be done in a number of ways, leading to a working diagnosis. Signs and symptoms alone may lead to a diagnosis, or the doctor may want to perform a number of diagnostic tests, including those offered by laboratory medicine. In some cases, the definitive diagnosis may become clear only over time as the nature of the condition evolves, or when a treatment is given (e.g., in the case of prescribing antibiotics in certain situations). In rare instances, a diagnosis is made only following death, with the aid of an autopsy. After a diagnosis, or working diagnosis, has been made, decisions can be made about the process of providing further care (e.g., to treat or not to treat). At this time, in some instances, the nature and severity of the condition will also be assessed to provide a prognosis. Each of these steps, as will be described later, represents a clinical problem (or clinical question) requiring a decision to be made—and action taken. The services of laboratory medicine are included among the tools at the disposal of the clinician to answer questions posed along this pathway, from initial hypothesis generation through to decision making.119 The tools of laboratory medicine are called diagnostic tests, but—as was mentioned earlier—these tests are used far more broadly than in making a diagnosis. As mentioned earlier and discussed later, they are also used in making a prognosis, excluding a diagnosis, monitoring a treatment or disease process, screening for disease, selecting therapy, evaluating the effects of therapy, and looking for side effects. The process of using a diagnostic test can be described as two processes: one at a macro level, and the other at a micro level. These are illustrated in Figures 4-1 and 4-2. EBLM is simply the application of principles and techniques of EBM to laboratory medicine. A clinician requesting an investigation has a question and needs to make a decision. The clinician hopes that the test result will help to answer the question and will assist in making the decision. Thus a definition of evidence-based laboratory medicine could be “the conscientious, judicious, and explicit use of best evidence in the use of laboratory medicine investigations for assisting in decision making about the care of individual patients.” It might also be expressed more directly in terms of health outcomes as “ensuring that the best evidence on testing is made available, and the clinician is assisted in using the best evidence to ensure that the best decisions are made about the care of the individual patient, and that the probability of improved health outcomes increases.” As discussed later, clearly the primary focus is on improving clinical outcomes, but in the delivery of the routine laboratory service, it is also important to consider the operational and economic impact of laboratory investigations.107,109,112 A good test is one that reliably answers the question being asked. To do this, the test has to meet three criteria, as will be described in other chapters of this book: (1) analytically the test has to meet accuracy and precision criteria (see Chapter 2), (2) the biological variability and other preanalytical criteria must be understood, and (3) given that the first two criteria are met, the test result must provide an answer to the question being asked; this is called diagnostic accuracy (see Chapter 3). However, having a good test is not sufficient. It has to be used properly, as part of an integrated pathway of care. This is often summarized as “ensuring the right patient, gets the right test, at the right time, that the right result is generated, the right decision is made, and the right action taken, in order that the right outcome can be delivered.”112 This is about ensuring that the use of a test leads to a better outcome. The basic tools required to practice EBLM can be summarized in the A5 cycle for EBLM (Figure 4-3). This EBLM cycle embraces five areas of activity related to the clinical problem112: • Asking or formulating the question that describes the problem • Acquiring the evidence that addresses the question • Appraising the evidence for relevance and quality • Applying the knowledge gained from the evidence in resolving the problem • Assessing or auditing the application to test the process of application, as well as the robustness of the knowledge However, this may not be enough, and it is helpful to know where and when these skills may be required (i.e., the context).112 Some examples of the scenarios in which EBLM skills have been applied are given in Table 4-1, and it is evident that a question is being asked in each of these scenarios—from patient through to policymaker. Referring back to the hypothetical patient, or care, pathway illustrated in Figure 4-1, a number of questions are being asked during the course of this journey. Obviously a patient does not necessarily progress down the whole pathway, as the problem may be resolved by a simple intervention at an early stage. The key clinical questions can be summarized as follows: • Does this patient have any evidence of the condition? A screening question • Does this patient have condition X? A “rule-in” diagnostic question • Can I rule out the patient having condition X? A “rule-out” question • What is this patient’s prognosis? A prognosis question • Will this treatment work for this patient? A treatment selection question • Have I got the dose right? A treatment optimization question • Is this treatment working for this patient? A treatment effectiveness question • Is the patient following the treatment protocol correctly? A treatment adherence question Clearly not every question applies in all situations. However, this is not the only perspective that should be considered in the commitment to delivering the highest quality of healthcare. Donabedian28 advocated an approach to assessing the quality of care based on structure, process, and outcome. So although the above may represent the main clinical questions, which for these purposes might be grouped under the heading of “Structure,” we have also to consider the questions surrounding process and outcomes, and how laboratory medicine services have an impact on these elements of healthcare delivery. We will return to these considerations later. These scenarios illustrate the importance of identifying the triad of question, decision, and action. Identifying these three components proves to be critical in designing studies of utility or outcomes of testing, as well in the critical appraisal of evidence. They are also important in audit (see later) of the use of investigations from the perspective of both clinical governance (clinical accountability) and financial governance (controlling the test demand in the context of economic governance). Recognition of this triad has led to the definition of an appropriate test request as one in which there is a clear clinical question for which the result will provide an answer, enabling the clinician to make a decision and initiate some form of action leading to a health benefit for the patient.108 In light of the earlier comment, this benefit could be extended to the health provider and to society as a whole to encompass more directly the potential for economic benefit. Examples of questions that specify the detail required to accurately qualify the use of a test result are given in Box 4-1. In practice, the clinical episode involves a series of diagnostic questions with binary responses.20 Reference was made earlier to the PICO framework for the formulation of an answerable question; in its general form, PICO comprises four elements, as illustrated in Box 4-2. A number of variants of this framework have been described. Given the patient pathway described in Figure 4-1 and the different stages at which a test might be used, the addition of “S” for setting has been suggested, giving PICOS or PSICO. The alternative is to qualify the “P” for population or patient according to setting, so that only data appropriate to that setting are considered. Clinicians who use probabilistic reasoning and likelihood ratios may wish to know the probability associated with the use of earlier tests or observations, and in this case “P” for prior test has been suggested, giving PPICO. Finally, time is an important consideration when tests are used (e.g., when looking at the prognostic accuracy of a test and the time over which a patient is observed after the laboratory test has been performed). The time at which samples are taken may also be important (e.g., when the effectiveness of digoxin therapy is monitored). This has led to the use of PICOT.112 Fryback and Thornbury40 developed a hierarchy of evidence in support of their thesis that patient outcome data are the sine qua non of efficacy from the individual patient’s perspective. This has been applied to laboratory medicine and is illustrated in Figure 4-4.107 Surveys of the literature, as well as many systematic reviews, have shown that many of the papers concerned with laboratory medicine, to date, have been concerned with technical (namely, analytical accuracy and precision) and diagnostic (namely, diagnostic accuracy) performance. Sackett and Haynes120 described an “architecture for diagnostic research,” which was based on four questions: • Do test results in affected patients differ from those in normal individuals? • Are patients with certain test results more likely to have the target disorder? • Do test results distinguish patients with and without the target disorder among those in whom it is clinically sensible to suspect the disorder? • Do patients undergoing the diagnostic test fare better than similar untested patients? When a new test is developed or an old test is applied to a new clinical question, users need information about the extent of agreement of the test’s results with the correct diagnoses of patients. For this purpose, researchers design studies in which results from the new test are compared with results obtained with the clinical reference standard on the same patients. Results of the comparison can be expressed in a number of ways, including clinical sensitivity and specificity, predictive values, likelihood ratios, diagnostic odds ratios, and areas under receiver operating characteristic (ROC) curves (see Chapter 3). We refer to such studies as diagnostic accuracy studies. The clinical reference standard is the best available method for establishing the presence or absence of the target disease or, more generally, the target condition, that is, the suspected condition or disease for which the test is to be applied. The reference standard can be a single test, or a combination of methods and techniques, including clinical follow-up of tested patients. When there is no clear reference procedure, it has been suggested that the best way to assess a new test is by analyzing the consequences when there is disagreement between the new tests and the test in current use.45 Finally, in some instances, the reference standard may consist of information obtained from an autopsy. Alternative designs are possible; Mol and associates have reviewed the characteristics of good studies of diagnostic tests.91 Some studies first select patients known to have the target condition, and then contrast the results from these patients with those from a control group. This approach has been used to characterize the performance of tests in settings in which the condition of interest is uncommon, as in maternal serum screening testing for detecting Down syndrome in the fetus. It is also used in preliminary studies to assess the potential of a test before prospective studies of a series of patients are undertaken. With this design, selection of the control group is critical. If the control group consists of healthy individuals only, the diagnostic accuracy of the test will tend to be overestimated, as has been shown in an analysis that compared the results of such studies with results of studies of consecutive series of patients.83 (See Chapter 3 for further discussion.) A different form of verification bias can happen if more than one reference standard is used and the two reference standards correspond to different manifestations of disease. The use of multiple standards can produce differential verification bias. Suppose test-positive patients are verified with further testing, and test-negative patients are verified by clinical follow-up. An example is the verification of suspected appendicitis, with histopathology of the appendix versus natural history as the two forms of the reference standard. A patient is classified as having a false-positive test result if the additional test does not confirm the presence of disease after a positive index test result. Alternatively, a patient is classified as false-negative if an event compatible with appendicitis is observed during follow-up after a negative test result. Yet these are different definitions of disease because not all patients who have positive pathology results would have experienced an event during follow-up if they had been left untreated. The use of two reference standards, one pathologic and the other based on clinical prognosis, can affect the assessment of diagnostic accuracy. It is likely to artificially inflate the estimates of accuracy, compared with the use of a single reference standard in all patients. For additional discussion, see Chapter 3. Severity of disease among studied patients with the target condition and the range of other conditions in those without the target condition can affect the apparent diagnostic accuracy of a test. For example, if a test that is designed to detect early cancer is evaluated in patients with clinically apparent cancer, the test is likely to perform better than when used for persons who do not yet show signs of the condition. This problem has been called spectrum bias and spectrum variation (see Chapter 3). Similarly, if a test is developed to distinguish diseased patients from those with similar complaints but without the target disease, then it may be misleading to use healthy subjects as controls when the diagnostic accuracy of the test is evaluated. Complete and accurate reporting of studies of diagnostic accuracy should allow the reader to detect the potential for bias in the study and to assess its ability to generalize the results and their applicability to an individual patient or group. Reid, Lachs, and Feinstein114 documented that most studies of diagnostic accuracy published in leading general medical journals had poor adherence to standards of clinical epidemiologic research or failed to provide information about adherence to those standards.7 Similar observations have continued to be made with a number of categories of tests.86 These reports led to efforts at the journal Clinical Chemistry in 1997 to produce a checklist for reporting of studies of diagnostic accuracy.15 The quality of reporting in that journal increased after introduction of this checklist,84 although not to an ideal level.11 The work of Lijmer and colleagues83 showed that poor study design and poor reporting are associated with overestimation of the diagnostic accuracy of evaluated tests, indicating the necessity to improve the reporting of studies of diagnostic accuracy for all types of tests, not only those in clinical chemistry. An initiative on Standards for Reporting of Diagnostic Accuracy (STARD) was begun at the 1999 meeting of the Cochrane Diagnostic and Screening Test Methods Working Group. This initiative aimed to improve the quality of reporting of diagnostic accuracy studies by following the model of the successful Consolidated Standards of Reporting Trials (CONSORT) initiative for reporting of trials of therapies (see discussion of outcomes studies later in this chapter).9 Key components of the STARD document include a checklist of items to be included in reports of studies of diagnostic accuracy and a flow diagram to document the flow of participants in the study.9 The checklist was developed from an extensive literature search that identified 75 potential items. The list was pared to 25 items (Figure 4-5) in a consensus meeting of researchers, editors, methodologists, and representatives of professional organizations. The flow diagram (Figure 4-6) has the potential to clearly communicate vital information about the design of a study—including the method of recruitment and the order of test execution—and about the flow of participants. The final, single-page checklist (see Figure 4-5) has been endorsed by numerous journals [such as Journal of the American Medical Association (JAMA) and Annals of Internal Medicine] and published in many of them, including all the major journals of clinical chemistry and other leading journals such as Radiology, British Medical Journal (BMJ), and Lancet. A separate document explaining the meaning and rationale of each item and briefly summarizing the available evidence was published in Annals of Internal Medicine and Clinical Chemistry.10 The STARD group will prepare updates of the STARD document when new evidence on sources of bias or variability becomes available. In the experience of one of the authors of this chapter (D.B.), use of the checklist has enhanced the information content of all manuscripts to which it has been applied at Clinical Chemistry, and use of the flow diagram has led to correction of errors in many manuscripts. Use of the STARD initiative is recommended for all reports on studies of diagnostic accuracy. Most, if not all, of the content of STARD also applies to studies of tests used for prognosis, monitoring, or screening. It is interesting to note that Simel and coworkers recently reported on use of the STARD approach for reporting of diagnostic accuracy of the history and physical examination133; this is important in that laboratorians need to be aware of the role that these play in the diagnostic armamentarium of the practicing physician.141 Smidt and associates136 found a small improvement in reporting, specifically, in reproducibility of the index test, in assessment of the severity of the condition and other diagnoses, and in estimates of variability of diagnostic accuracy between subgroups. On the other hand, Wilczynski found no improvement to date.151 In the area of laboratory medicine, the objective can be described in terms of answering a clinical question; appropriate laboratory investigations help to answer the question.108 Knowledge of the characteristics of these investigations is needed to decide which test to use, when to use it, and how to interpret the results. Analytical performance of the test may also have an impact on the outcome of the use of that test, although this is not a factor that is commonly studied. Some information has come from modeling studies, as for example in the case where the impact of the accuracy and precision of blood glucose tests on insulin dosage has been calculated.13 The impact of the accuracy and precision of total prostate-specific antigen (PSA) methods on the number of cancers detected and of biopsies required has also been modeled.118 Differences in analytical performance can have an impact on outcomes through differences in the decisions that are made. At the postanalytical stage, if a result is not received or accessed, then clearly it cannot contribute to an improved outcome. In a systematic study, Kilpatrick and Holding72 found that when they introduced electronic transmission of data to the emergency department and admissions unit, a notable number of results were never accessed. In another study of POCT for HbA1c, Khunti and colleagues after consultation merely replaced the phlebotomy service with the POCT, with no apparent immediate discussion of the result,71 thus omitting the key objective for introducing the POCT.18 Medical and public health interventions are intended to improve the well-being of patients, the population at large, or population segments, as stated by Fryback and Thornbury40 and Sackett and Haynes.120 For therapeutic interventions, patients are interested, for example, not only in whether a drug decreases serum cholesterol or blood pressure (risk factors), but more importantly, whether it decreases the risks of heart attack, stroke, and cardiovascular death. Similarly, on the diagnostic side of medicine, patients have little interest in knowing the numeric value of their serum cholesterol concentration or blood pressure unless that knowledge will lead to actions that in some way will improve their quality or quantity of life. For example, a test result may identify the need for a life-saving therapeutic intervention for an existing disease, or it may lead to a change in lifestyle that will decrease the risk of developing a disease. At other times, the test result itself can provide valuable reassurance, as when a genetic test indicates that a family member does not carry a mutation that is present in the family. In still other cases, a laboratory test may provide prognostic information that allows the patient to better plan for the future despite a bad prognosis, or it may provide reassurance that symptoms are not signs of serious disease, thus allowing him or her to better manage the symptoms without fear. Test-related outcomes in these examples range from preventing imminent death to being better able to plan for death. In the United States, the important Joint Commission defines quality as increased probability of desired outcomes and decreased probability of undesired outcomes. The Institute of Medicine defines quality as “the degree to which health services for individuals and populations increase the likelihood of desired health outcomes and are consistent with current professional knowledge.”127 Up until this point, the focus has been on the patient and his or her interaction with the doctor as the primary caregiver; these individuals may be considered as the two primary stakeholders. Yet we know that healthcare is the product of many healthcare professionals, sometimes referred to as the clinical team. The complexity of interactions in this group has to be extended, when the broader issues of healthcare delivery are considered, to include the roles of service provider managers, service purchasers or commissioners, and policymakers. This has been brought more sharply into focus with increased pressure to improve the quality of care and to make more efficient and effective use of resources.61,62,98 So in the case of laboratory medicine, the identity of stakeholders should be extended to include all of those who have an interest in how the laboratory service can be, and is, used. In many clinical scenarios, the first criterion for a useful test is that the result must lead to a change in the probability of the presence of the target condition. Boyd and Deeks,14 for example, showed that the (pretest) probability of pulmonary embolism fell from about 0.28 to a post-test probability of 0.041 when the D-dimer test result was less than 500 µg/L. The change in probability does not, in itself, make the decision. The clinician must use this information along with other findings and clinical judgment to make decisions or recommendations about care. So, even the knowledge that a test has good diagnostic accuracy is of no value unless it is used correctly, and results are integrated with other observations and the expertise of the clinician.140 In most cases, testing is followed by an appropriate intervention to produce a desired outcome, particularly when the outcome is defined as improved morbidity or mortality. A test result alone may provide reassurance or an understanding of the origin of one’s complaint, but usually improved outcomes require an intervention in the form of an explanation of the result in the context of the patient’s symptoms. Most laboratory medicine research encompasses only test characteristics, including diagnostic accuracy. If these characteristics are not linked to clear consequences for downstream decisions and interventions, such research leads to poor understanding and appreciation of the contribution that the test result makes to improved outcomes. In relation to certain scenarios, it is possible to find valid study data, most particularly when the test result is being used to exclude a diagnosis. Thus in a randomized study of a rapid chest pain evaluation protocol, cardiac markers had a high negative predictive value in the evaluation of patients with chest pain. Testing led to fewer admissions to the coronary care unit, without adverse effects on morbidity and mortality.99 Reducing the turnaround time for certain tests may improve triage time in the emergency room82 unless other evaluations (such as other laboratory tests or radiologic investigations) are rate limiting.69 Reduced length of stay appeared to occur primarily because normal results with the POCT approach enabled some patients to be discharged more quickly (i.e., a rule-out decision).95 These examples are process outcomes.
Evidence-Based Laboratory Medicine
Concepts, Definitions, and Relationships
What Is Laboratory Medicine?
What is Evidence-Based Medicine?
Definitions, Concepts, and Objectives of Evidence-Based Medicine
The Practice of EBM
Evidence-Based Medicine and Laboratory Medicine
What Is Evidence-Based Laboratory Medicine?
The Practice of Evidence-Based Laboratory Medicine
Types of Questions Addressed in Laboratory Medicine
Formulating an Answerable Question in Laboratory Medicine
From Evidence to Outcomes
Characterization of the Diagnostic Accuracy of Tests
Study Design
Reporting of Studies of Diagnostic Accuracy: The Role of the STARD Initiative
Using the Test Result
Outcome Studies
Who Is Interested in Health Outcomes?
Test Results Alone Do Not Generate Improved Health Outcomes
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


Basicmedical Key
Fastest Basicmedical Insight Engine