THERAPEUTIC AND TOXIC CONCENTRATIONS
The therapeutic range for ethosuximide is defined by most laboratories as 40-100 μg/mL, although some clinicians suggest drug concentrations as high as 150 μg/mL with appropriate monitoring of serum concentrations and possible side effects.9 The most common adverse effects of ethosuximide are gastric distress, nausea, vomiting, and anorexia, but these gastrointestinal problems appear to be due to local irritation of gastric mucosa. Generally, administration of smaller doses and more frequent dosing of the drug produce relief from these side effects. In the upper end of the therapeutic range (>70 μg/mL) some patients will begin to experience the concentration-dependent adverse effects of ethosuximide treatment: drowsiness, fatigue, lethargy, dizziness, ataxia, hiccups, euphoria, and headaches. Idiosyncratic side effects that are independent of concentration include rash, systemic lupus-like syndromes, and blood dyscrasias (leukopenia, pancytopenia).
CLINICAL MONITORING PARAMETERS
The goal of therapy with anticonvulsants is to reduce seizure frequency and maximize quality of life with a minimum of adverse drug effects. While it is desirable to entirely abolish all seizure episodes, it may not be possible to accomplish this in many patients. Patients should be monitored for concentration-related side effects (drowsiness, fatigue, lethargy, dizziness, ataxia, hiccups, euphoria, headaches) as well as gastrointestinal upset associated with local irritation of gastric mucosa (gastric distress, nausea, vomiting, anorexia). Serious, but rare, idiosyncratic side effects include systemic lupus-like syndromes, leukopenia, and pancytopenia.
Ethosuximide serum concentrations should be measured in most patients. Because epilepsy is an episodic disease state, patients do not experience seizures on a continuous basis. Thus, during dosage titration it is difficult to tell if the patient is responding to drug therapy or simply is not experiencing any abnormal central nervous system discharges at that time. Ethosuximide serum concentrations are also valuable tools to avoid adverse drug effects. Patients are more likely to accept drug therapy if adverse reactions are held to the absolute minimum.
BASIC CLINICAL PHARMACOKINETIC PARAMETERS
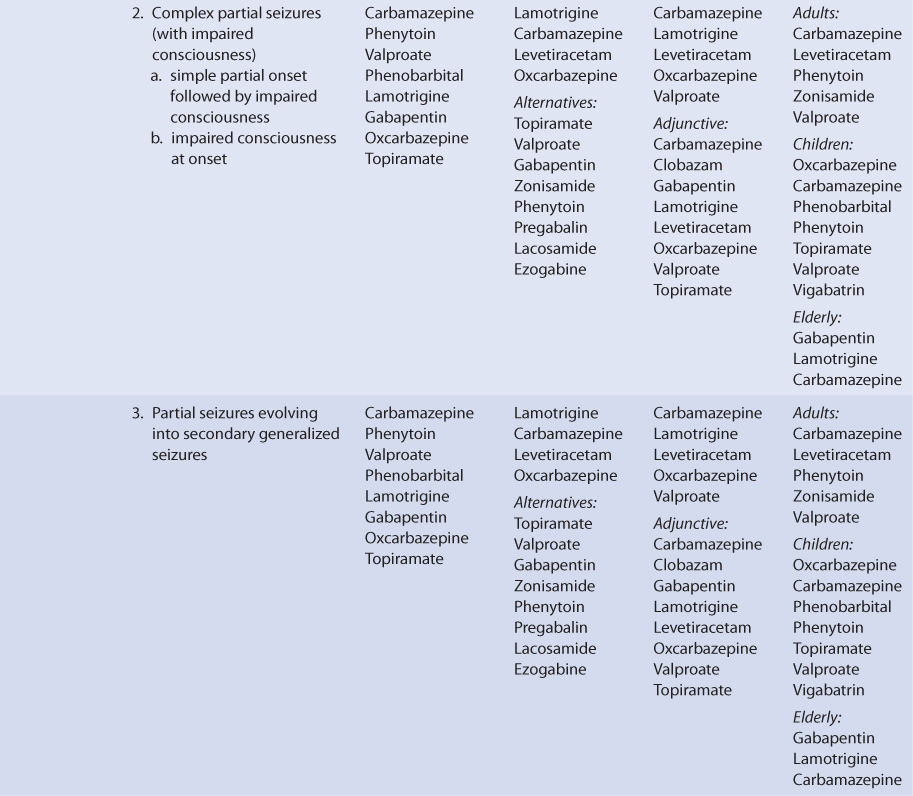
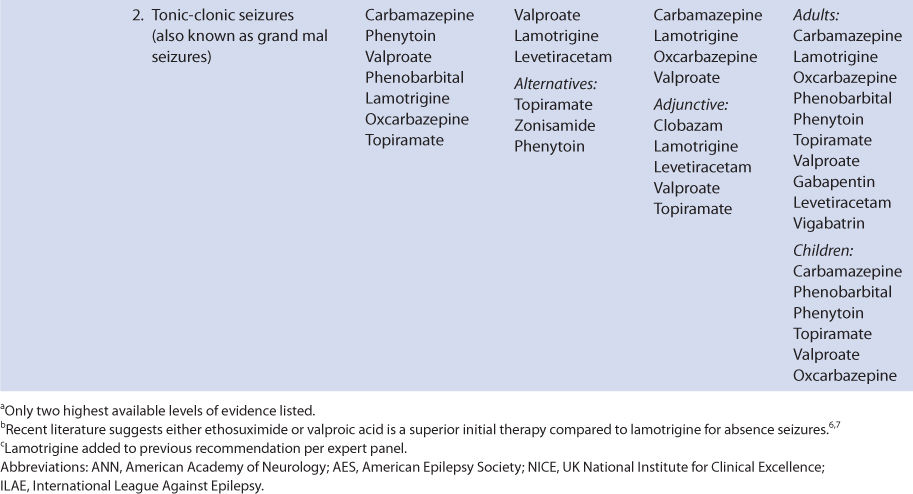
Ethosuximide is eliminated primarily by hepatic metabolism (70%-80%) via hydroxylation by CYP3A4 and then conjugated to inactive metabolites.10,11 About 20%-30% of a ethosuximide dose is recovered as unchanged drug in the urine.12 Ethosuximide is not significantly bound to plasma proteins. At concentrations exceeding 100 μg/mL, the drug may follow nonlinear pharmacokinetics, presumably due to Michaelis-Menten (concentration-dependent or saturable) metabolism.13 Because an intravenous form of the drug is not commercially available, the absolute bioavailability in humans is not known. However, based on animal studies, ethosuximide oral bioavailability of capsules (250 mg) and syrup (250 mg/5 mL) is assumed to be 100%.9 The typical maintenance dose for ethosuximide is 20 mg/kg/d for pediatric patients (<12 years old) and 15 mg/kg/d for older patients.9
EFFECTS OF DISEASE STATES AND CONDITIONS ON PHARMACOKINETICS AND DOSING
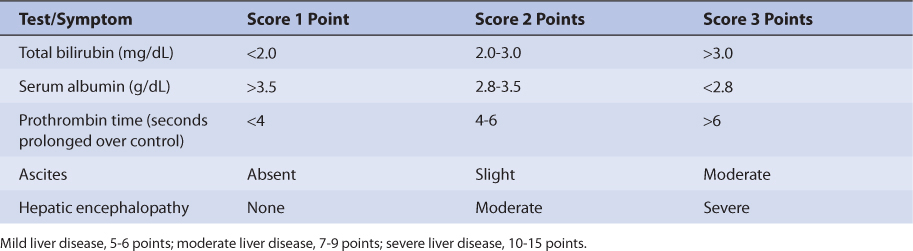
Ethosuximide oral clearance rate (Cl/F) for older children (≥12 years old) and adults is 12 mL/h/kg and for children is 16 mL/h/kg.9 Ethosuximide volume of distribution (V/F) equals 0.7 L/kg, and its half-life averages 30 hours in children and 60 hours in adults.9 Although studies in patients with hepatic disease are not available, 70%-80% of the drug is eliminated by hepatic metabolism. Because of this, patients with liver cirrhosis or acute hepatitis may have reduced ethosuximide clearance because of destruction of liver parenchyma. This loss of functional hepatic cells reduces the amount of enzymes available to metabolize the drug and decreases clearance. An index of liver dysfunction can be gained by applying the Child-Pugh clinical classification system to the patient (Table 14-2).14 Child-Pugh scores are completely discussed in Chapter 3 (Drug Dosing in Special Populations: Renal and Hepatic Disease, Dialysis, Heart Failure, Obesity, and Drug Interactions), but will be briefly discussed here. The Child-Pugh score consists of five laboratory tests or clinical symptoms: serum albumin, total bilirubin, prothrombin time, ascites, and hepatic encephalopathy. Each of these areas is given a score of 1 (normal) to 3 (severely abnormal; see Table 14-2), and the scores for the five areas are summed. The Child-Pugh score for a patient with normal liver function is 5 while the score for a patient with grossly abnormal serum albumin, total bilirubin, and prothrombin time values in addition to severe ascites and hepatic encephalopathy is 15. A Child-Pugh score greater than 8 is grounds for a decrease of 25%-50% in the initial daily drug dose for ethosuximide. As in any patient with or without liver dysfunction, initial doses are meant as starting points for dosage titration based on patient response and avoidance of adverse effects. Ethosuximide serum concentrations and the presence of adverse drug effects should be monitored frequently in patients with liver cirrhosis.
Similarly, a small amount (20%-30%) of ethosuximide is usually eliminated unchanged by the kidneys so patients with renal dysfunction (creatinine clearance <30 mL/min) receiving ethosuximide should be closely monitored.12 Ethosuximide is significantly removed by hemodialysis, and supplemental doses may need to be given after a dialysis session.15 The drug crosses into the placenta and enters breast milk, achieving concentrations at both sites similar to concurrent maternal serum concentrations.16–18
DRUG INTERACTIONS
Unlike other antiepileptic drugs, ethosuximide is not a hepatic enzyme inducer or inhibitor, and appears to cause no clinically important drug interactions.19 Valproic acid can inhibit ethosuximide metabolism and increase steady-state concentrations, especially when ethosuximide serum concentrations are in the upper end of the therapeutic range.13
INITIAL DOSAGE DETERMINATION METHODS
Several methods to initiate ethosuximide therapy are available. The Pharmacokinetic Dosing method is the most flexible of the techniques. It allows individualized target serum concentrations to be chosen for a patient, and each pharmacokinetic parameter can be customized to reflect specific disease states and conditions present in the patient. Literature-based Recommended dosing is a very commonly used method to prescribe initial doses of ethosuximide. Doses are based on those that commonly produce steady-state concentrations in the lower end of the therapeutic range, although there is a wide variation in the actual concentrations for a specific patient.
Pharmacokinetic Dosing Method
The goal of initial dosing of ethosuximide is to compute the best dose possible for the patient, given their set of disease states and conditions that influence ethosuximide pharmacokinetics and the epileptic disorder being treated. In order to do this, pharmacokinetic parameters for the patient will be estimated using average parameters measured in other patients with similar disease state and condition profiles.
Clearance Estimate
Ethosuximide is predominately metabolized by liver. Unfortunately, there is no good way to estimate the elimination characteristics of liver-metabolized drugs using an endogenous marker of liver function in the same manner that serum creatinine and estimated creatinine clearance are used to estimate the elimination of agents that are renally eliminated. Because of this, a patient is categorized according to the disease states and conditions that are known to change ethosuximide clearance, and the clearance previously measured in these studies is used as an estimate of the current patient’s clearance. For example, for a 20-kg pediatric patient, ethosuximide clearance would be assumed to equal 16 mL/h/kg: 20 kg • 16 mL/h/kg = 320 mL/h or 0.32 L/h. To produce the most conservative ethosuximide doses in patients with multiple concurrent disease states or conditions that affect ethosuximide pharmacokinetics, the disease state or condition with the smallest clearance should be used to compute doses. This approach will avoid accidental overdosage has much as currently possible.
Volume of Distribution Estimate
Ethosuximide volume of distribution is assumed to equal 0.7 L/kg for adults and children. Thus, for a 20-kg pediatric patient, the estimated ethosuximide volume of distribution would be 14 L: V = 0.7 L/kg • 20 kg = 14 L.
Half-Life and Elimination Rate Constant Estimate
Once the correct clearance and volume of distribution estimates are identified for the patient, they can be converted into the ethosuximide half-life (t1/2) and elimination rate constant (k) estimates using the following equations: t1/2 = (0.693 • V)/Cl, k = 0.693/t1/2 = Cl/V.
Selection of Appropriate Pharmacokinetic Model and Equations
Ethosuximide follows a one-compartment pharmacokinetic model. When oral therapy is required, ethosuximide has good bioavailability (F = 1), and once or twice dosing provides a relatively smooth serum concentration-time curve that emulates an intravenous infusion. Because of this, a very simple pharmacokinetic equation that computes the average ethosuximide steady-state serum concentration (Css in μg/mL = mg/L) is widely used. This allows maintenance dosage calculation: Css = [F(D/τ)]/Cl or D = (Css • Cl • τ)/F, where F is the bioavailability fraction for the oral dosage form (F = 1 for oral ethosuximide products), D is the dose of ethosuximide in mg, Cl is ethosuximide clearance in L/h, and τ is the dosage interval in hours.
Literature-Based Recommended Dosing
Because of the large amount of variability in ethosuximide pharmacokinetics, even when concurrent disease states and conditions are identified, most clinicians believe that the use of standard ethosuximide doses for various situations is warranted. The original computation of these doses was based on the Pharmacokinetic Dosing methods, and was subsequently modified based on clinical experience. In general, the expected ethosuximide steady-state serum concentrations used to compute these doses was 40-50 μg/mL. The usual initial maintenance dose for pediatric patients (<12 years old) is 20 mg/kg/d. For older patients, the initial maintenance dose is 15 mg/kg/d. One or two divided daily doses are initially used for these total doses. To avoid gastrointestinal side effects, doses over 1500 mg given at one time should be avoided. Dosage increases of 3-7 mg/kg/d are made every 1-2 weeks depending on response and adverse effects. While maximal doses are 40 mg/kg/d for children less than 12 years old and 30 mg/kg/d for older patients, ethosuximide serum concentrations and adverse effects should be used to judge optimal response to the drug. If the patient has significant hepatic dysfunction (Child-Pugh score ≥8), maintenance doses prescribed using this method should be decreased by 25%-50% depending on how aggressive therapy is required to be for the individual.
To illustrate the similarities and differences between this method of dosage calculation and the Pharmacokinetic Dosing method, the same examples used in the previous section will be used.
USE OF ETHOSUXIMIDE SERUM CONCENTRATIONS TO ALTER DOSES
Because of the large amount of pharmacokinetic variability among patients, it is likely that doses computed using patient population characteristics will not always produce ethosuximide serum concentrations that are expected or desirable. Because of pharmacokinetic variability, the possible nonlinear pharmacokinetics followed by the drug at high concentrations, the narrow therapeutic index of ethosuximide, and the desire to avoid adverse side effects of ethosuximide, measurement of ethosuximide serum concentrations is conducted for most patients to ensure that therapeutic, nontoxic levels are present. In addition to ethosuximide serum concentrations, important patient parameters (seizure frequency, potential ethosuximide side effects, etc) should be followed to confirm that the patient is responding to treatment and not developing adverse drug reactions.
When ethosuximide serum concentrations are measured in patients and a dosage change is necessary, clinicians should seek to use the simplest, most straightforward method available to determine a dose that will provide safe and effective treatment. In most cases, a simple dosage ratio can be used to change doses since ethosuximide follows Linear Pharmacokinetics. Sometimes, it is not possible to simply change the dose because of the limited number of oral dosage strengths, and the dosage interval must also be changed. In some situations, it may be necessary or desirable to compute the ethosuximide pharmacokinetic parameters for the patient and utilize these to calculate the best drug dose. Computerized methods that incorporate expected population pharmacokinetic characteristics (Bayesian pharmacokinetic computer programs) can be used in difficult cases where renal function is changing, serum concentrations are obtained at suboptimal times, or the patient was not at steady-state when serum concentrations were measured. An additional benefit of this method is that a complete pharmacokinetic workup (determination of clearance, volume of distribution, and half-life) can be done with one or more measured concentrations that do not have to be at steady-state.
Linear Pharmacokinetics Method
Because ethosuximide follows linear, dose-proportional pharmacokinetics in most patients with concentrations within and below the therapeutic range, steady-state serum concentrations change in proportion to dose according to the following equation: Dnew/Css,new = Dold/Css,old or Dnew = (Css,new/Css,old)Dold, where D is the dose, Css is the steady-state concentration, old indicates the dose that produced the steady-state concentration that the patient is currently receiving, and new denotes the dose necessary to produce the desired steady-state concentration. The advantages of this method are that it is quick and simple. The disadvantages are that steady-state concentrations are required and the assumption of linear pharmacokinetics may not be valid in all patients. When steady-state serum concentrations increase more than expected after a dosage increase or decrease less than expected after a dosage decrease, nonlinear ethosuximide pharmacokinetics is a possible explanation for the observation. Because of this, suggested dosage increases greater than 75% while using this method should be scrutinized by the prescribing clinician, and the risk versus benefit for the patient should be assessed before initiating large dosage increases (>75% over current dose).