Ethanol and Methanol
The 2-carbon alcohol ethanol (CH3CH2OH), or beverage alcohol, is one of the most versatile drugs known to man, with multiple direct effects on a diverse range of neurochemical systems. Produced in nature, rewarding in its effects, and easy to manufacture, it has been taken by humans since the beginning of recorded history, is consumed by a large majority of people in the Western world, and is likely to contribute to more morbidity, mortality, and public health costs than all of the illicit drugs combined.
ETHANOL CONSUMPTION. Compared with other drugs, surprisingly large amounts of alcohol are required for physiological effects, resulting in its consumption more as a food than a drug. The alcohol content of beverages typically ranges from 4-6% (volume/volume) for beer, 10-15% for wine, and 40% and higher for distilled spirits (the “proof” of an alcoholic beverage is twice its percentage of alcohol; e.g., 40% alcohol is 80 proof). A glass of beer or wine, a mixed drink, or a shot of spirits contains ~14 g alcohol, or ~0.3 mol ethanol. Thus, alcohol is consumed in gram quantities, whereas most other drugs are taken in milligram or microgram doses.
Because the ratio of ethanol in end-expiratory alveolar air and ethanol in the blood is relatively consistent, blood ethanol concentrations (BECs) in humans can be estimated readily by the measurement of alcohol levels in expired air; the partition coefficient for ethanol between blood and alveolar air is approximately 2000:1. Because of the causal relationship between excessive alcohol consumption and vehicular accidents, there has been a near-universal adoption of laws attempting to limit the operation of vehicles while under the influence of alcohol. Legally allowed BECs in the U.S. typically are set at or below 80 mg% (80 mg ethanol per 100 mL blood; 0.08% w/v), which is equivalent to a concentration of 17 mM ethanol in blood. A 12-oz bottle of beer, a 5-oz glass of wine, and a 1.5-oz “shot” of 40% liquor each contains approximately 14 g ethanol, and the consumption of 1 of these beverages by a 70-kg person would produce a BEC of ~30 mg%. However, it is important to note that this is approximate because the BEC is determined by a number of factors, including the rate of drinking, gender, body weight and water percentage, and the rates of metabolism and stomach emptying (see “Acute Ethanol Intoxication” later in the chapter).
PHARMACOLOGICAL PROPERTIES
ETHANOL
ABSORPTION. After oral administration, ethanol is absorbed rapidly into the bloodstream from the stomach and small intestine and distributes into total-body water (0.5-0.7 L/kg). Peak blood levels occur about 30 min after ingestion of ethanol when the stomach is empty. Because absorption occurs more rapidly from the small intestine than from the stomach, delays in gastric emptying (owing, e.g., to the presence of food) slow ethanol absorption. Because of first-pass metabolism by gastric and liver alcohol dehydrogenase (ADH), oral ingestion of ethanol leads to lower BECs than would be obtained if the same quantity were administered intravenously. Gastric metabolism of ethanol is lower in women than in men, which may contribute to the greater susceptibility of women to ethanol. Aspirin increases ethanol bioavailability by inhibiting gastric ADH.
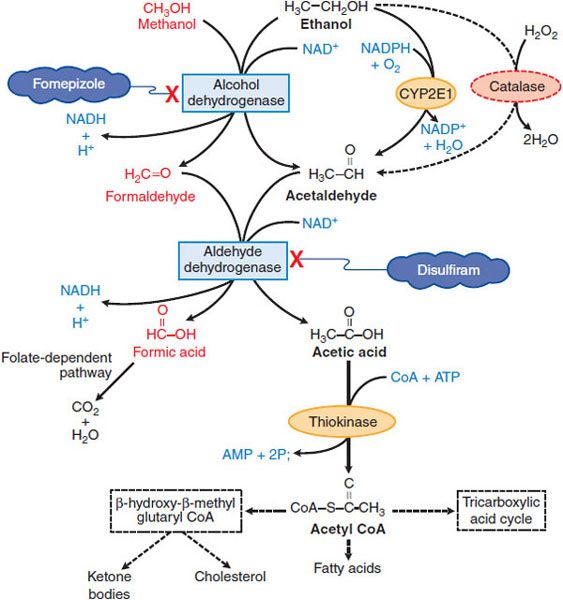
METABOLISM. Ethanol is metabolized largely (90-98%) by sequential hepatic oxidation, first to acetaldehyde by ADH and then to acetic acid by aldehyde dehydrogenase (ALDH) (Figure 23–1). Each metabolic step requires NAD+; this greatly exceeds the supply of NAD+ in the liver and NAD+ availability limits ethanol metabolism to ~8 g or 10 mL (~170 mmol) per hour in a 70-kg adult, or ~120 mg/kg/h. Thus, hepatic ethanol metabolism functionally saturates at relatively low blood levels compared with the high BECs achieved, and ethanol metabolism is a zero-order process (constant amount per unit time). Small amounts of ethanol are excreted in urine, sweat, and breath.
Figure 23–1 Metabolism of ethanol and methanol.
CYP2E1 also can contribute, especially at higher ethanol concentrations. CYP2E1 is induced by chronic consumption of ethanol, increasing the clearance of its substrates and the activation of certain toxins such as CCl4. There can be decreased clearance of the same drugs, however, after acute consumption of ethanol because ethanol competes with them for oxidation by the enzyme system (e.g., phenytoin and warfarin).
The large increase in the hepatic NADH:NAD+ ratio during ethanol oxidation has profound consequences in addition to limiting the rate of ethanol metabolism. Enzymes requiring NAD+ are inhibited; thus, lactate accumulates, activity of the tricarboxylic acid cycle is reduced, and acetyl coenzyme A (acetyl CoA) accumulates (and it is produced in quantity from ethanol-derived acetic acid; see Figure 23–1). The combination of increased NADH and elevated–acetyl CoA supports fatty acid synthesis and the storage and accumulation of triacylglycerides; ketone bodies accrue, exacerbating lactic acidosis. Ethanol metabolism by the CYP2E1 pathway produces elevated NADP+, limiting the availability of NADPH for the regeneration of reduced glutathione (GSH), thereby enhancing oxidative stress.
Genetic Variation in Ethanol Metabolism. The enzymes involved in ethanol metabolism are mainly ADH and ALDH, and secondarily, catalase and CYP2E1. CYPs 1A2 and 3A4 may also participate. Several of these enzymes have genetic variants that alter alcohol metabolism and susceptibility to its effects.
The genetics of the ADH isoforms are important for understanding risk factors for severe repetitive ethanol problems. The 3 relevant forms are ADH1A, 1B, and 1C. These class I ADHs have Km<34 mmol (0.15 g/dL) and are responsible for 70% of the ethanol metabolizing capacity at BECs of 22 mM (i.e., ~0.10 g/dL). These ADH forms are the rate-limiting step in ethanol metabolism, reducing the BECs by ~4-5 mM (0.015-0.020 g/dL) per hour, the approximate levels of alcohol resulting from the consumption of 1 standard drink.
The ADH1A gene has no polymorphisms known to significantly affect the rate of alcohol metabolism. The ADH1B gene has a polymorphism, ADH1B*2, with arginine 47 replaced by histidine to produce a variant form of ADH with a 40-fold higher Vmax than ADH1B. This polymorphism is seen in 30-45% of Chinese, Japanese, and Koreans, less than 10% of most Europeans, but in 50-90% of Russians and Jews. The potential faster metabolism of ethanol may result in a transient slightly higher blood level of acetaldehyde and is reported to be associated with a lower risk for heavy drinking and ethanol-related problems. A second polymorphism for ADH1B, ADH1B*3 (arginine 269 replaced by cysteine), has a 30-fold higher Vmax. ADHlB*3 is seen in about 30% of Africans and also is associated with lower risk of heavy drinking and ethanol problems.
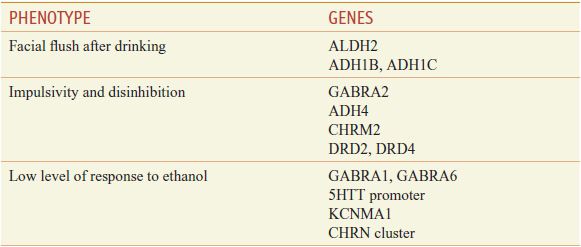
Acetaldehyde is produced from the breakdown of ethanol at the rate of approximately 1 standard drink per hour. As shown in Figure 23–1, the acetaldehyde is then rapidly broken down through the actions of ALDH2, primarily in the mitochondria of liver cells. The actions of ALDH2 are important because low levels of acetaldehyde may be perceived as rewarding and stimulating, while high blood levels of this substance produce severe adverse reactions that can include vomiting, diarrhea, and unstable blood pressure. There is a mutation in the ALDH2 gene (12q24), ALDH2*2 (resulting from a substitution of glycine 487 with lysine). Homozygotes with a nonfunctional ALDH2*2 occur in 5-10% of Japanese, Chinese, and Korean individuals, for whom severe adverse reactions occur after consumption of 1 drink or less. This reaction operates through the same mechanism that occurs with drinking after taking the ALDH2 inhibitor, disulfiram. Heterozygotes for this polymorphism (ALDH2*2, 2*1) make up 30-40% of Asian individuals who, after consuming ethanol experience a facial flush and an enhanced sensitivity to beverage alcohol, but who do not necessarily report an overall adverse response to the drug. A number of these polymorphisms affect risk for alcohol use disorder (Table 23–1).
Table 23–1
Genes for Intermediate Phenotypes Affecting Risk for Alcohol Use Disorder
METHANOL
Methanol (CH3OH), is also known as methyl and wood alcohol. It is an important industrial reagent and solvent found in products such as paint removers, shellac, and antifreeze; methanol is added to industrial-use ethanol to mark it unsafe for human consumption.
Absorption and Metabolism. Methanol is rapidly absorbed via the oral route, inhalation, and through the skin, with the latter 2 routes most relevant to industrial settings. Methanol is metabolized by ADH and ALDH. Competition between methanol and ethanol for ADH forms the basis of the use of ethanol in methanol poisoning. Several drugs inhibit alcohol metabolism, including fomepizole (4-methylpyrazole), an ADH inhibitor useful in ethylene glycol poisoning, and disulfiram, an ALDH inhibitor used in treating alcoholism.
Feelings of intoxication from methanol, while similar in many ways to those with ethanol, are less intense, and often delayed by 8 or more hours from ingestion, progressing even more slowly if methanol is taken along with ethanol. As little as 15 mL of methanol can produce toxicity, including blindness, with doses in excess of 70 mL capable of producing death. Methanol poisoning consists of headache, GI distress, and pain (partially related to pancreatic injury), difficulty breathing, restlessness, and blurred vision associated with hyperemic optic disks. Severe metabolic acidosis can develop due to the accumulation of formic acid, and the respiratory depression can be severe, especially in the context of coma. The visual disturbances occur as a consequence of injury to ganglion cells of the retina by the metabolite, formic acid, with subsequent inflammation, atrophy, and potential bilateral blindness. The clinical picture can also include necrosis of the pancreas.
EFFECTS OF ETHANOL ON PHYSIOLOGICAL SYSTEMS
William Shakespeare described the acute pharmacological effects of imbibing ethanol in the Porter scene (act 2, scene 3) of Macbeth. The Porter, awakened from an alcohol-induced sleep by Macduff, explains three effects of alcohol and then wrestles with a fourth effect that combines the contradictory aspects of soaring overconfidence with physical impairment:
Porter: … and drink, sir, is a great provoker of three things.
Macduff: What three things does drink especially provoke?
Porter: Marry, sir, nose-painting [cutaneous vasodilation], sleep [CNS depression], and urine [a consequence of the inhibition of antidiuretic hormone (vasopressin) secretion, exacerbated by volume loading]. Lechery, sir, it provokes and unprovokes: it provokes the desire but it takes away the performance. Therefore much drink may be said to be an equivocator with lechery: it makes him and it mars him; it sets him on and it takes him off; it persuades him and disheartens him, makes him stand to and not stand to [the imagination desires what the corpus cavernosum cannot deliver]; in conclusion, equivocates him in a sleep, and, giving him the lie, leaves him.
More recent research has added details to Shakespeare’s enumeration—see the bracketed additions to the Porter’s words in the preceding paragraph and the section on organ systems later in the chapter—but the most noticeable consequences of the recreational use of ethanol still are well summarized by the gregarious and garrulous Porter, whose delighted and devilish demeanor demonstrates frequently observed influences of modest concentrations of ethanol on the CNS.
CENTRAL NERVOUS SYSTEM
Although the public often views alcoholic drinks as stimulating, ethanol primarily is a CNS depressant. Ingestion of moderate amounts of ethanol, like that of other depressants such as barbiturates and benzodiazepines, can have antianxiety actions and produce behavioral disinhibition at a wide range of dosages. Individual signs of intoxication vary from expansive and vivacious affect to uncontrolled mood swings and emotional outbursts that may have violent components. With more severe intoxication, CNS function generally is impaired, and a condition of general anesthesia ultimately prevails. However, there is little margin between the anesthetic actions and lethal effects (usually owing to respiratory depression).
Chronic alcohol abuse is accompanied by tolerance, dependence, and craving for the drug. Alcoholism is characterized by compulsive use despite clearly deleterious social and medical consequences. Alcoholism is a progressive illness, and brain damage from chronic alcohol abuse contributes to the deficits in cognitive functioning and judgment seen in alcoholics. Alcoholism is a leading cause of dementia in the U.S. Chronic alcohol abuse results in shrinkage of the brain owing to loss of both white and gray matter. In addition to loss of brain tissue, alcohol abuse also reduces brain metabolism, and this hypometabolic state rebounds to a level of increased metabolism during detoxification. The magnitude of decrease in metabolic state is determined by the number of years of alcohol use and the age of the patients.
Actions of Ethanol on Neurochemical Pathways and Signaling. Ethanol affects almost all brain systems. The changes across neural pathways occur simultaneously and the alterations often interact. An additional complication in describing CNS effects is the rapid adaptation to ethanol observed in the brain, with the result that the acute effects of the first dose of ethanol are often the opposite of the neurochemical consequences from repeated administration and those observed during falling blood ethanol levels and withdrawal syndromes. Alcohol perturbs the balance between excitatory and inhibitory influences in the brain, resulting in anxiolysis, ataxia, and sedation. This is accomplished by either enhancing inhibitory or antagonizing excitatory neurotransmission. The 12th edition of the parent text summarizes research supporting effects of ethanol on a number of ion channels and neurotransmitter signal transducing systems that alter neuronal excitability within the CNS.
Ethanol Consumption and CNS Function. Large doses of ethanol can interfere with encoding of memories, producing anterograde amnesias, commonly referred to as alcoholic blackouts; affected individuals are unable to recall all or part of experiences during the period of heavy intake. Perhaps reflecting the effect of ethanol on respirations as well as the muscle-relaxant effects of this drug, heavier drinking can be associated with sleep apnea, especially in older alcohol-dependent subjects. The transient CNS effects of heavy ethanol consumption that produce a hangover—the “next morning” syndrome of headache, thirst, nausea, and cognitive impairment—may reflect mechanisms similar to mild alcohol withdrawal, dehydration, and/or mild acidosis.
Chronic heavy drinking reportedly increases the probability of developing alcoholic dementia
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree