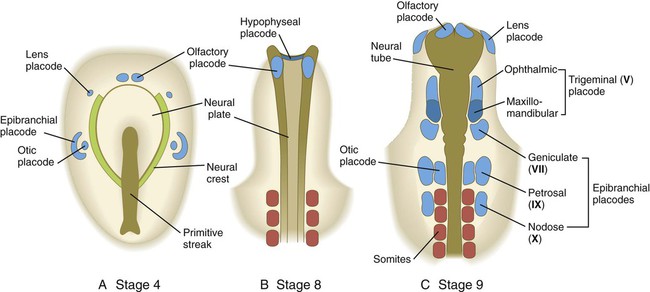
As seen in Chapter 5, despite the relatively featureless appearance of the gastrulating embryo, complex patterns of gene expression set up the basic body plan of the embryo. One of the earliest morphological manifestations of this pattern is the regular segmentation that becomes evident along the craniocaudal axis of the embryo. Such a segmental plan, which is a dominant characteristic of all early embryos, becomes less obvious as development progresses. Nonetheless, even in an adult the regular arrangement of the vertebrae, ribs, and spinal nerves persists as a reminder of humans’ highly segmented phylogenetic and ontogenetic past. This chapter concentrates on the establishment of the basic overall body plan. In addition, it charts the appearance of the primordia of the major organ systems of the body from the undifferentiated primary germ layers (see Fig. 6.27). The principal early morphological response of the embryonic ectoderm to neural induction is an increase in the height of the cells that are destined to become components of the nervous system. These transformed cells are evident as a thickened neural plate visible on the dorsal surface of the early embryo (Figs. 6.1A and 6.2A). Unseen but also important is the restricted expression of cell adhesion molecules (Ig-CAMs), from N-CAM and E-cadherin in the preinduced ectoderm to N-CAM and N-cadherin in the neural plate. The first of four major stages in the formation of the neural tube is transformation of the general embryonic ectoderm into a thickened neural plate. The principal activity of the second stage is further shaping of the overall contours of the neural plate so that it becomes narrower and longer. To a great extent, this is accomplished by convergent extension, during which the ectodermal cells forming the neural plate migrate toward the midline and also become longer along the anteroposterior axis and narrower laterally. This process, which is guided by planar cell polarity (see p. 87), results in the formation of a key-shaped neural plate (see Fig. 6.1A). The third major stage in the process of neurulation is the lateral folding of the neural plate that results in the elevation of each side of the neural plate along a midline neural groove (see Figs. 6.1B and 6.2B). Many explanations have been proposed for lateral folding of the neural plate and ultimate closure of the neural tube. Most of these explanations have invoked a single or dominant mechanism, but it is now becoming apparent that lateral folding is the result of numerous region-specific mechanisms intrinsic and extrinsic to the neural plate. The ventral midline of the neural plate, sometimes called the median hinge point, acts like an anchoring point around which the two sides become elevated at a sharp angle from the horizontal. At the median angle, bending can be largely accounted for by notochord-induced changes in the shape of the neuroepithelial cells of the neural plate. These cells become narrower at their apex and broader at their base (see Fig. 6.2B) through a combination of a basal position of the nuclei (thus causing a lateral expansion of the cell in that area) and a purse string–like contraction of a ring of actin-containing microfilaments in the apical cytoplasm. Throughout the lateral folding of the neural plate in the region of the spinal cord, much of the wall area of the neural plate initially remains flat (see Fig. 6.2B), but in the brain region, a lateral hinge point forms as a result of apical constriction of cells in a localized area (see Fig. 6.2C). Elevation of the neural folds seems to be accomplished largely by factors extrinsic to the neural epithelium, in particular, pushing forces generated by the expanding surface epithelium lateral to the neural plate. The fourth stage in the formation of the neural tube consists of apposition of the two most lateral apical surfaces of the neural folds, their fusion (mediated by cell surface glycoconjugates), and the separation of the completed segment of the neural tube from the overlying ectodermal sheet (see Fig. 6.2C and D). At the same time, cells of the neural crest begin to separate from the neural tube. Closure of the neural tube begins almost midway along the craniocaudal extent of the nervous system of a 21- to 22-day-old embryo (see Fig. 6.1C). Over the next couple of days, closure extends caudally in a zipperlike fashion, but cranially there are commonly two additional discontinuous sites of closure. The unclosed cephalic and caudal parts of the neural tube are called the anterior (cranial) and posterior (caudal) neuropores. The neuropores also ultimately close off so that the entire future central nervous system resembles an irregular cylinder sealed at both ends. Occasionally, one or both neuropores remain open, and serious birth defects result (see p. 248). Soon after the neural tube has taken shape, the region of the future brain can be distinguished from the spinal cord. The brain-forming region undergoes a series of subdivisions that constitute the basis for the fundamental gross organization of the adult brain. Segmentation by subdivision of an existing structure (in the case of the neural tube) contrasts with segmentation by adding terminal segments, as is the case in the formation of somites (see p. 99). An early set of subdivisions results in a three-part brain, consisting of a forebrain (prosencephalon), midbrain (mesencephalon), and hindbrain (rhombencephalon). Later, the prosencephalon becomes subdivided into a telencephalon and diencephalon, and the rhombencephalon is subdivided into a metencephalon and myelencephalon (see Fig. 11.2). Superimposed on the traditional gross morphological organization of the developing brain is another, more subtle, level of segmentation, which subdivides certain regions of the brain into transiently visible series of regular segments called neuromeres (Fig. 6.3). In the hindbrain, the neuromeres, often called rhombomeres, are visible from early in the fourth to late in the fifth week (Fig. 6.3B). The midbrain does not seem to be segmented, but the prosencephalon contains a less regular series of prosomeres. Rhombomeres are arranged as odd and even pairs, and when established, they act like isolated compartments in insect embryos. Because of specific surface properties, cells from adjacent rhombomeres do not intermingle across boundaries between even and odd segments; however, marked cells from two even or two odd rhombomeres placed side by side do intermingle. During their brief existence, rhombomeres provide the basis for the fundamental organization of the hindbrain. In an adult, the segmental organization of the rhombomeres is manifest in the rhombomere-specific origin of many cranial nerves and parts of the reticular formation within the brainstem (see Fig. 11.13). While gastrulation is still taking place, the newly induced neural tube is subjected to vertical inductions from the notochord and head organizing regions (anterior visceral endoderm and prechordal plate), which are important in inducing the forebrain region. These inductions, together with a gradient of Wnt-8 (product of a gene homologous with Wingless, a segment polarity gene in Drosophila [see Fig. 4.1]) signaling, effectively subdivide it into forebrain/midbrain and hindbrain/spinal cord segments. This subdivision is marked by the expression of two transcription factors, Otx-2 (orthodenticle homologue 2) in the forebrain/midbrain region, and in the hindbrain, Gbx-2 (gastrulation brain homeobox 2), whose boundaries sharply define the midbrain-hindbrain border (Fig. 6.4A). Fibroblast growth factors (FGFs), produced in the early primitive streak, are known to exert a posteriorizing effect on the newly forming neural plate. The midbrain-hindbrain border becomes a powerful local signaling center, called the isthmic organizer. Wnt-1 is synthesized in the neural ectoderm anterior, and FGF-8 is formed posterior to the isthmic organizer (Fig. 6.4B). The transcription factors Pax-2 and Pax-5 and engrailed (En-1 and En-2) are expressed on both sides of the isthmic organizer as gradients that are crucial in organizing the development of the midbrain and the cerebellum, a hindbrain derivative. Two additional organizing or signaling centers are established early in the formation of the forebrain region. One, the anterior neural ridge, is located at the anterior pole of the brain (see Fig. 6.4B). It is a site of sonic hedgehog and FGF-8 signaling activity and is important in organizing the formation of the telencephalon, parts of the diencephalon, the olfactory area, and the pituitary gland. A third signaling center, the zona limitans (see Fig. 6.4B), is a sonic hedgehog–secreting group of cells that organize the border between the future dorsal and ventral thalamus. Chapter 11 presents additional information on the organization and segmentation of the forebrain. Segmentation of the hindbrain into seven rhombomeres in humans, and eight in some other animals, is the result of the expression of several categories of genes, which operate in a manner remarkably reminiscent of the way in which the early Drosophila embryo becomes subdivided into segments (see Fig. 4.1). Individual rhombomeres are initially specified through the ordered expression of unique combinations of transcription factors; this patterning is then translated into cellular behavior by the patterned expression of cell surface molecules. After the Gbx-2–expressing area defines the rough limits of the hindbrain, several segmentation genes are involved in setting up the basic pattern of segmentation that leads to rhombomere formation. Krox 20, a zinc finger transcription factor, is expressed in and guides the formation of rhombomeres 3 and 5 (r3 and r5) (see Fig. 11.12), whereas kreisler, another transcription factor, and Hoxa-1 are also involved in the formation of r5. A decreasing gradient of retinoic acid, produced by the anterior somites, plays an important role in the formation of the posterior rhombomeres (r4 to r7). These molecules are not involved in the specification of r1 to r3, which is regulated by Gbx-2. The Hox genes are principally involved in specifying segmental identity, but before any molecular marker of morphological segmentation exists, the previously mentioned gradient of retinoic acid stimulates the expression of Hoxa-1 and Hoxb-1. The influence of these two Hox genes and of the segmentation genes, Krox 20 and kreisler, initiates the expression of the various Hox paralogues in a highly specific sequence along the hindbrain and spinal cord (see Fig. 11.12). As seen in Chapters 11 and 14, the pattern of Hox gene expression determines the morphological identity of the cranial nerves and other pharyngeal arch derivatives that arise from specific rhombomeres. At successive times during the formation of the hindbrain, different regulatory networks controlling Hox gene expression come into play, but details of these networks are not presented in this text. The orderly expression of Hox gene paralogues extends anteriorly through r2. Hox proteins are not found in r1 largely because of the antagonistic action of FGF-8, which is produced in response to signals from the isthmic organizer at the anterior end of r1. In the absence of FGF-8, Hox proteins are expressed in r1. Another rhombencephalic protein, sprouty 2, acts as an antagonist of FGF-8, and this protein, in addition to the presence of Hoxa-2 in r2, confines FGF-8 mostly to r1 and contains the primordium of the cerebellum to the anterior part of r1. Another family of genes, the ephrins and their receptors, determines the behavioral properties of the cells in the rhombomeres. The action of ephrins, which are expressed in even-numbered rhombomeres (2, 4, and 6), and of ephrin receptors, which are expressed in odd-numbered rhombomeres (3 and 5), seems to account for the lack of mixing behavior of cells from adjacent rhombomeres and maintains the separation of the various streams of neural crest cells that emigrate from the rhombomeres (see Fig. 12.8). As the body axis is elongating, and somites are forming, the caudalmost part of the newly induced neural plate possesses the properties of a stem cell zone (Fig. 6.5). Under the influence of FGF-8, secreted by the adjacent presomitic paraxial mesoderm, these cells, which go on to form the spinal cord, proliferate without undergoing differentiation. Some of the daughter cells are left behind by the posteriorly advancing stem cell zone. These cells fall under the influence of retinoic acid, produced by the newly formed somites, which are also being formed in a posterior direction (see Fig. 6.8). Retinoic acid stimulates these cells to differentiate into neurons. Elongation of the tail bud region comes to a close when the caudal extent of presomitic mesoderm is reduced, thus allowing the retinoic acid produced in the area to diffuse farther posteriorly and inhibit the action of FGF-8. As a result, proliferation of tail bud mesenchyme is greatly reduced, causing growth to cease. The opposing actions of retinoic acid, which promotes differentiation, and FGF, which fosters proliferation at the expense of differentiation, represent a recurring theme in the development of other structures. For example, the spread of FGF-8 from the isthmic organizer (see Fig. 6.4B) antagonizes the influence of retinoic acid in r1. This permits the exuberant proliferation of the cells in this rhombomere, which is necessary for the formation of the large cerebellum from this structure. Interactions between FGF-8 and retinoic acid in the forming spinal cord and paraxial mesoderm help to set the Hox code that confers anteroposterior identity to regions of the spinal cord and the adjacent somites. As the neural tube is closing and separating from the general cutaneous ectoderm, a population of cells called the neural crest leaves the dorsal part of the neural tube and begins to spread throughout the body of the embryo (see Fig. 6.2). The neural crest produces an astonishing array of structures in the embryo (see Table 12.1), and its importance is such that the neural crest is sometimes called the fourth germ layer of the body. (The neural crest is discussed further in Chapter 12.) As the cranial region begins to take shape, several series of ectodermal placodes (thickenings) appear lateral to the neural tube and neural crest (Fig. 6.6). These placodes arise from a horseshoe-shaped preplacodal domain around the anterior neural plate that is established during the gastrulation and early neurulation periods, and the individual placodes result from a variety of secondary inductive processes between neural or mesenchymal tissues and the overlying ectoderm (see Table 13.1). In several cases, cells from the placodes and neural crest interact closely to form the sensory ganglia of cranial nerves (V, VII, IX, and X). Deficiencies of one of these two components can often be made up by an increased contribution by the other component. Further details of placodes and their developmental fate are given in Chapter 13. After passing through the primitive streak, the mesodermal cells spread laterally between the ectoderm and endoderm as a continuous layer of mesenchymal cells (see Fig. 5.6). Subsequently, three regions can be recognized in the mesoderm of cross-sectioned embryos (Fig. 6.7B). Nearest the neural tube is a thickened column of mesenchymal cells known as the paraxial mesoderm, or segmental plate. This tissue soon becomes organized into somites. Lateral to the paraxial mesoderm is a compact region of intermediate mesoderm, which ultimately gives rise to the urogenital system. Beyond that, the lateral plate mesoderm ultimately splits into two layers and forms the bulk of the tissues of the body wall, the wall of the digestive tract, and the limbs (see Fig. 6.27). As the primitive node and the primitive streak regress toward the caudal end of the embryo, they leave behind the notochord and the induced neural plate. Lateral to the neural plate, the paraxial mesoderm appears to be a homogeneous strip of closely packed mesenchymal cells. However, if scanning electron micrographs of this mesoderm are examined with stereoscopic techniques, a series of regular pairs of segments can be discerned. These segments, called somitomeres, have been most studied in avian embryos, but they are also found in mammals. New pairs of somitomeres form along the primitive node as it regresses toward the caudal end of the embryo (Fig. 6.8). Not until almost 20 pairs of somitomeres have formed, and the primitive node has regressed quite far caudally, does the first pair of somites (brick-shaped masses of paraxial mesoderm) form behind the seventh pair of somitomeres. After the first pair of somites has been established (approximately 20 days after fertilization), a regular relationship develops between the regression of the primitive streak and the formation of additional somites and somitomeres. The first 7 pairs of somitomeres in the cranial region do not undergo further separation or segmentation. Cells from these somitomeres (cranial mesoderm) will form most of the skeletal musculature of the head, and they have quite different cellular and molecular properties from those derived from somites of the trunk. The first pair of somites forms at the expense of the eighth pair of somitomeres. In the types of embryos studied to date, there is a constant relationship between the caudalmost pair of definitive somites and the number of somitomeres (usually 10 to 11) that can be shown behind them. Every few hours, the pair of somitomeres located caudal to the last-formed somites becomes transformed into a new pair of somites, and a new pair of somitomeres is laid down at the caudal end of the paraxial mesoderm near the primitive node (see Fig. 6.8). As regression of the primitive streak comes to a close, the formation of paraxial mesoderm continues through the cells contributed by the tail bud. The cervical, thoracic, and lumbar vertebrae and associated structures are derived from cells migrating through the primitive streak, whereas the cellular precursors of the sacrum and coccyx come from the tail bud. The formation of individual somites from a seemingly homogeneous strip of paraxial mesoderm is a complex process that involves a variety of levels of molecular control and changes in cellular behavior within the paraxial mesoderm. Our basic understanding of somitogenesis (somite formation) comes from studies on the chick. The first significant step in somitogenesis is segmentation of the paraxial mesoderm. In contrast to segmentation in the hindbrain (see p. 95), somite formation occurs by the sequential addition of new segments in a craniocaudal sequence. Somitogenesis involves two mechanisms in what is often referred to as a clock and wavefront model. The first step (the wavefront) is associated with the elongation of the caudal end of the body through proliferative activity of mesenchymal cells in the most posterior nonsegmented part of the primitive streak (Fig. 6.9A). Cells in this area divide actively under the influence of a high local concentration of FGF-8. More anteriorly, where the cells are older, the concentration of FGF-8 decreases as the FGF molecules become broken down over time. Conversely, the cells closer to the last-formed somite become exposed to increasing concentrations of retinoic acid, which is produced in the most posterior somites and whose action opposes that of FGF. At some point in their life history, the mesenchymal cells are exposed to a balance of FGF-8 and retinoic acid concentrations that results in their crossing a developmental threshold (the wavefront, or determination front) that prepares them for entering the process of segmentation (somite formation). This is characterized by the expression of a transcription factor, Mesp-2, which prefigures a future somite. With the continued caudal elongation of the embryo and the addition of new somites, the location of the wavefront extends caudally in the growing embryo, but it remains a constant distance from the last-formed somite pair. Next, the segmentation clock is initiated in those presomitic cells that have passed over the previously mentioned threshold and are expressing Mesp-2. The exact mechanism that starts the clock is still not fully defined, but many molecules in the interacting Notch, Wnt, and FGF pathways are known to be synthesized at regular periodic intervals and become localized at critical locations in the forming somite. In the chick, in which a new somite forms every 90 minutes, lunatic fringe becomes concentrated at the future anterior border of the somite, and c-hairy (a homologue of a segmentation gene in Drosophila) becomes concentrated along the future posterior border (Fig. 6.9B). At the level of cellular behavior, cells at the anterior border of the forming somite express the ephrin receptor Eph A. Because the cells on the posterior border of the previously formed somite express the ephrin ligand ephrin B, the cells of the two adjacent somites are prevented from mixing (as is the case with adjacent rhombomeres in the developing hindbrain), and a fissure forms between the two somites. Finally, the action of Wnt-6 from the overlying ectoderm stimulates the expression of the transcription factor paraxis in the newly forming somite. This, along with the downregulation of Snail, results in the transformation of the mesenchymal cells of the anterior part of the somite, and later all the mesenchymal cells, into an epithelial cell type (Fig. 6.10A
Establishment of the Basic Embryonic Body Plan
Development of the Ectodermal Germ Layer
Neurulation: Formation of the Neural Tube

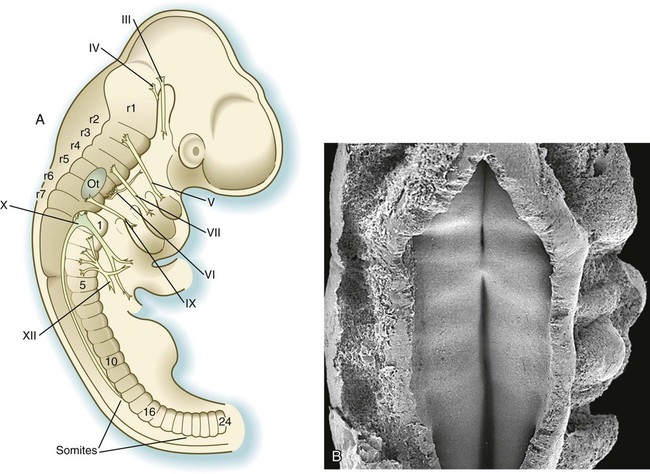
A, At 18 days. B, At 20 days. C, At 22 days. D, At 23 days.
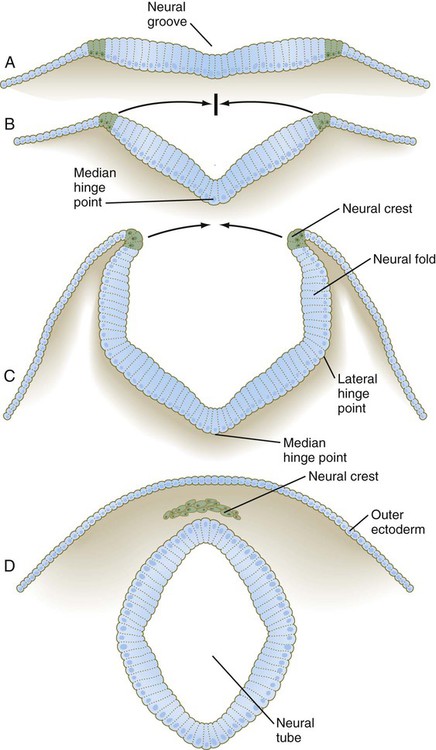
A, Neural plate. B, Neural fold. C, Neural folds apposing. D, Neural tube complete. (Neural crest before and after its exit from the neural epithelium is shown in green.)
Segmentation in the Neural Tube
Morphological Manifestations of Segmentation
Mechanisms of Early Segmentation of the Neural Tube
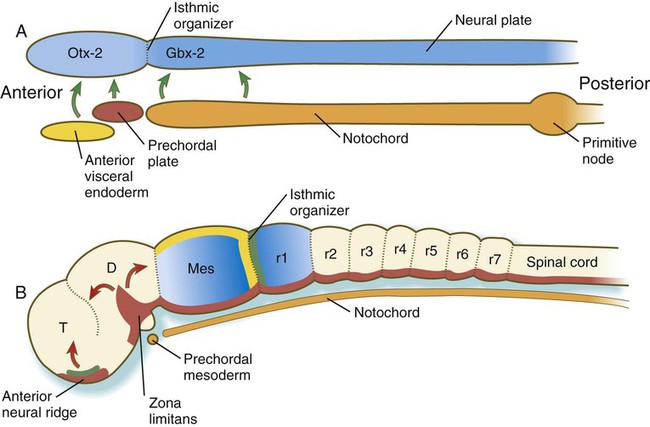
A, In response to signals (green arrows) from the anterior visceral endoderm, the prechordal plate, and the notochord, the neural tube expresses Otx-2 in the future forebrain and midbrain regions and Gbx-2 in the hindbrain and spinal cord. B, Later in development, signals (fibroblast growth factor-8 [FGF-8] [green] and Wnt-1 [yellow]) from the isthmic organizer induce decreasing gradients of En-1 and En-2 (blue) on either side. Another organizer—the anterior neural ridge—secretes sonic hedgehog (red) and FGF-8 (green), and both the zona limitans and the ventral part (floor plate) of the neural tube secrete sonic hedgehog. D, diencephalon; Mes, mesencephalon; r, rhombomere; T, telencephalon. (B, After Lumsden A, Krumlauf R: Science 274:1109-1115, 1996.)
Segmentation in the Hindbrain Region
Formation and Segmentation of the Spinal Cord
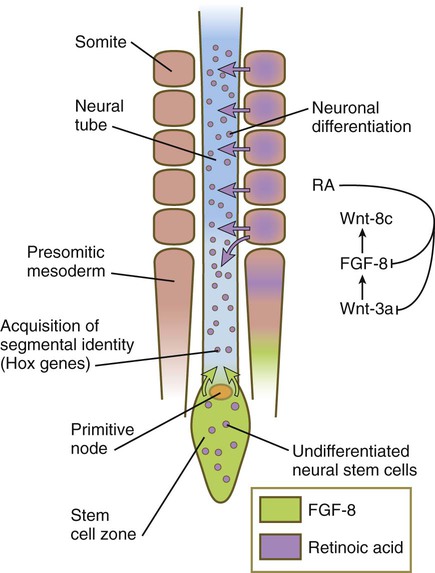
Under the influence of fibroblast growth factor-8 (FGF-8) secreted by presomitic paraxial mesoderm, cells in the most posterior region continue to proliferate, whereas retinoic acid (RA), secreted by newly formed somites, stimulates the neuronal differentiation.
Neural Crest
Sensory Placodes and Secondary Inductions in the Cranial Region
Development of the Mesodermal Germ Layer
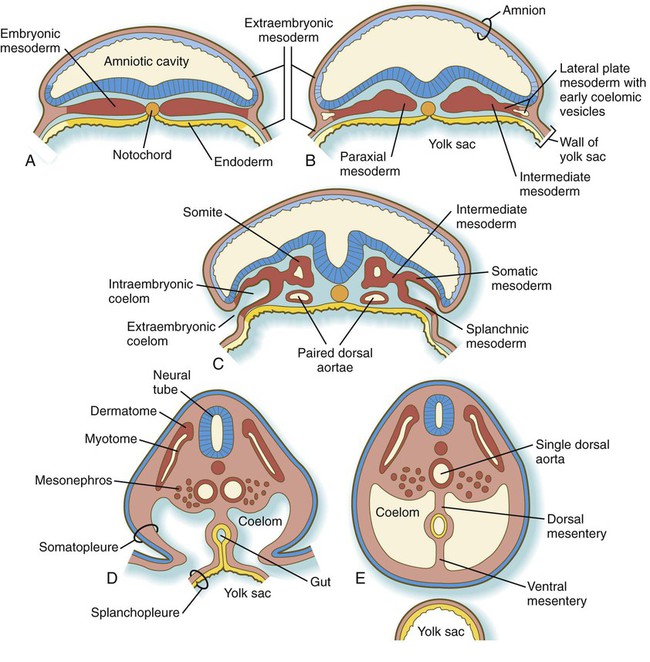
Basic Plan of the Mesodermal Layer
Paraxial Mesoderm
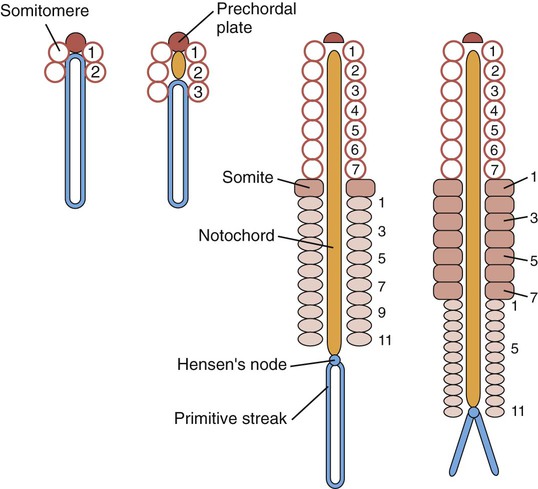
Cranial somitomeres (open circles) take shape along Hensen’s node until 7 pairs have formed. Caudal to the seventh somitomere, somites (rectangles) form from caudal somitomeres (ovals). As the most anterior of the caudal somitomeres transform into somites, additional caudal somitomeres take shape posteriorly. For a while, the equilibrium between transformation into somites anteriorly and new formation posteriorly keeps the number of caudal somitomeres at 11.
Formation of Individual Somites
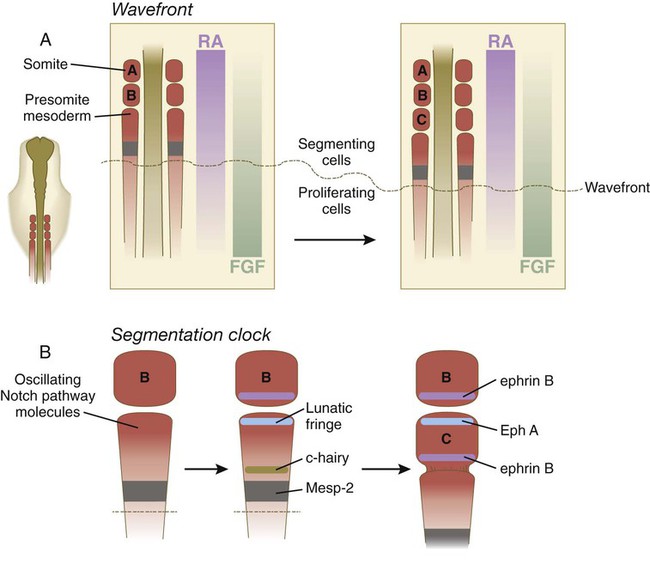
A, The wavefront, consisting of opposing gradients of retinoic acid (RA) and fibroblast growth factor-8 (FGF). B, The segmentation clock, in which oscillating molecules in the Notch pathway stimulate the expression of lunatic fringe at the anterior and c-hairy at the posterior border of a future somite. Later interactions between Eph A and ephrin B maintain the intersomitic space.
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


Establishment of the Basic Embryonic Body Plan