The underlying lamina propria consists of fibrovascular tissue folded into slender papillae that project into the epithelium. These papillae generally extend to less than two-thirds of the epithelial thickness, except in the distal 2 to 3 cm of the esophagus where mild elongation may normally be found. The lamina propria contains capillaries, lymphatic vessels, elastic fibers, and scattered mononuclear cells and lymphoid aggregates; these should not be interpreted as evidence of esophagitis. Indigenous mucus-secreting glands (cardiac glands) are also found within the lamina propria, particularly along the distal esophagus. These glands, which produce neutral mucins, resemble the mucous glands of the stomach and may contain a few parietal or chief cells.
The junction between squamous and columnar mucosa represents the squamocolumnar junction or Z-line. The squamocolumnar junction does not necessarily coincide with the anatomic gastroesophageal junction (the site at which the tubular esophagus joins the first fold of the saccular stomach). Numerous studies have found that many adult patients have metaplastic cardiac-type mucosa in the distal esophagus (up to 2 to 3 cm proximal to the gastroesophageal junction) as a consequence of physiologic or pathologic gastroesophageal reflux (4–9). Thus, biopsy specimens procured from the most distal portion of the esophagus frequently show inflamed cardiac-type mucosa. As discussed in the following texts, unless intestinal metaplasia (goblet cells) is identified, the identification of this type of mucosa is not diagnostic of Barrett esophagus. Foci of pancreatic acinar metaplasia can also be seen in the area of the gastroesophageal junction, although this represents an incidental, presumably congenital, occurrence without clinical significance (10).
The submucosa is represented by a wide layer of loose connective tissue bearing blood vessels, lymphatics, nerve fibers, and scattered lymphocytes and lymphoid aggregates. In addition, there is a population of tubuloalveolar mucous glands that are dispersed throughout the esophagus. These submucosal glands, which secrete acidic mucins and are analogous to the minor salivary glands of the oropharynx, drain through cuboidal or squamous-lined ducts that obliquely penetrate the mucosa.
The muscularis propria is distinguished by a screw-like arrangement of both smooth and striated muscle fibers fashioned into inner circular and outer longitudinal layers. Striated muscle predominates in the uppermost esophagus, but it soon yields to smooth muscle, which alone forms the distal half of the musculature (1). Each end of the esophagus is demarcated by a sphincter. The upper esophageal sphincter is an anatomically distinct structure composed of fibers of the cricopharyngeus and inferior pharyngeal constrictor muscles. The lower esophageal sphincter, on the other hand, lacks a single anatomic counterpart and is instead formed from the intrinsic muscles of the distal esophagus, the sling fibers of the proximal stomach, and the crural diaphragm. Together, these structures create a functional zone of increased intraluminal pressure (11). A serosal lining covers short segments of the thoracic and abdominal esophagus, but most of the organ is instead ensheathed by an adventitia of loose connective tissue.
SPECIMEN HANDLING
Surgical pathologists most often encounter the esophagus through mucosal biopsy specimens. The typical endoscopic biopsy is obtained with large-capacity radial jaw forceps with needle that grasps and avulses a fragment of the mucosa. The resulting specimen, usually 1 to 5 mm in diameter, includes the epithelium with a variable amount of lamina propria and occasionally a slip of muscularis mucosae. For assessment of submucosal lesions, a jumbo forceps is used.
The biopsy specimens ideally should be mounted at the time of procurement by placing them, submucosal side down, on a supporting medium such as filter paper, nylon mesh, or Gelfoam and then placing them immediately in fixative, thus facilitating proper orientation during embedding and sectioning. When mounting procedures are not available, the specimen can be promptly dropped unmounted into fixative, although the orientation may be less than optimal. Standard formalin fixation generally suffices, although picric acid fixatives or mercury-based fixatives are preferred by some pathologists. Multiple sections at several levels in the paraffin block are necessary because of the focal nature of many lesions; this can also help obviate the lack of precise orientation in unmounted specimens. Serial sectioning is not necessary or practical, but retaining extra sections from each level for possible special stains or other procedures can be helpful.
Endoscopic mucosal resection (EMR), also called endoscopic submucosal dissection or mucosectomy, is an increasingly common specimen from the esophagus and requires specific handling to ensure adequate assessment of deep and lateral margins. The specimen may be received oriented by the surgeon, but regardless, the deep soft tissue should be inked and the specimen should be serially sectioned such that any potentially positive lateral margins would be perpendicular at the edge of the section.
Esophagectomy specimens are best handled by opening longitudinally, preferably in the fresh state, and then pinning them flat and fixing overnight. Directed attention can then be given to the size, appearance, and anatomic relationships of any lesions and appropriate blocks obtained to evaluate their nature and extent. The status of the resection margins, including the radial or adventitial margin deep to tumors, must be established; lymph nodes in the periesophageal adipose tissue should also be carefully dissected out and submitted; and involvement of any adjacent structures should be documented.
The handling and reporting of esophageal biopsy and resection specimens have been comprehensively reviewed elsewhere (12–14).
ESOPHAGITIS
The most common diagnostic problems encountered with esophageal biopsy specimens involve the evaluation of esophagitis and its consequences. A general approach to examining these biopsies entails identifying the histologic features of esophagitis, searching for clues to the cause, and appraising possible complications.
In a broad sense, esophagitis is recognized by the presence of epithelial damage and inflammation. Although this recognition is straightforward when mucosal exudation, erosion, or ulceration is present, these findings denote more severe degrees of esophagitis. They can be absent in low-grade epithelial injury, including that caused by gastroesophageal reflux, so that attention also needs to be given to other less conspicuous indicators of epithelial injury and repair. The term acute esophagitis is often employed to indicate this spectrum of changes, but because it ambiguously carries both temporal and morphologic connotations, the term active esophagitis is employed here instead.
Esophagitis can be caused by diverse agents—physical, chemical, and biologic—but by far the most common culprit is gastroesophageal reflux, with infectious organisms holding a distant second place. The precise cause, however, may not be apparent from biopsy examination; correlation with clinical, radiologic, and endoscopic details is necessary before a final diagnosis can be rendered. Occasionally, however, the biopsy reveals features diagnostic or suggestive of a specific cause (Table 31.1). These findings should be carefully sought, particularly when warranted by a pertinent clinical setting.

Other reasons for examining esophageal specimens are to investigate the long-term consequences of persistent or recurrent esophagitis: fibrosis (with the possibility for stricture formation), chronic ulceration, Barrett esophagus, and carcinoma.
GASTROESOPHAGEAL REFLUX DISEASE
Reflux of gastric contents into the esophagus is the preeminent cause of esophagitis. Although reflux episodes occur in healthy individuals, abnormal gastroesophageal reflux can cause clinical and pathologic manifestations that are described under the generic label of gastroesophageal reflux disease (GERD). Reflux esophagitis is often used to specifically designate the histopathologic changes that occur secondary to GERD. Exactly how physiologic reflux becomes the pathologic process of GERD is incompletely understood, but numerous complex factors are likely involved, including the volume and potency of the refluxate, the duration of esophageal exposure, and the efficiency of esophageal defense mechanisms (15–17).
Epidemiologic studies defining GERD as heartburn and/or acid regurgitation occurring at least weekly suggest a 20% prevalence of GERD in the United States as compared to 10% to 18% in Europe and 3% to 5% in Asia (18). The initial diagnosis of GERD is often based on clinical symptoms, most often heartburn, regurgitation, or dysphagia, and many patients will have decreased symptoms by taking a proton pump inhibitor (PPI), also called the PPI test. However, the PPI test has poor specificity (19), and response to PPIs does not always correlate with objective measures of GERD, including pH monitoring and endoscopy with biopsy (20,21). Also, symptoms may occur without other positive diagnostic tests, and, conversely, abnormal endoscopic and pathologic findings may be found in asymptomatic patients (22,23). Consequently, there is currently no completely sensitive and specific gold standard for the diagnosis of GERD.
Endoscopic examination is commonly used to evaluate patients with suspected GERD who have severe clinical symptoms, fail empiric PPI therapy, or have risk factors for esophageal carcinoma (24). The appearances may be clearly diagnostic: linear or punctate erosions or ulcerations, often with attendant inflammatory exudation, that occur predominantly in the distal esophagus (25). Other less dramatic changes such as mucosal erythema or edema can also be seen, but these are subject to greater observer variation and thus are diagnostically less conclusive. Moreover, up to 50% of patients with documented symptomatic GERD can display normal or only minimally abnormal endoscopic findings (endoscopy-negative reflux disease or nonerosive reflux disease [NERD]) (23). Mucosal biopsy in these cases can aid in establishing the diagnosis (26).
The histologic abnormalities of GERD encompass a range of features denoting epithelial injury and repair (Table 31.2). No individual or group of histologic features is diagnostic of GERD. Each feature is limited to some degree by practical use or diagnostic sensitivity or specificity, and although these numerous features, alone or in combination, can contribute to establishing the diagnosis of GERD, none is an absolutely reliable criterion (27–29). Multiple biopsy specimens sectioned at several levels provide the best diagnostic opportunity because the histologic alterations are known to be unevenly distributed both circumferentially and axially (30). Also, the balance of evidence seems to indicate that the presence and severity of reflux changes decreases with increasing distance from the squamocolumnar junction (31,32) and, therefore, more distal biopsies may be more sensitive. Most importantly, none of the histologic changes seen in GERD are specific for reflux; all can be seen in other causes of esophagitis (Table 31.1).
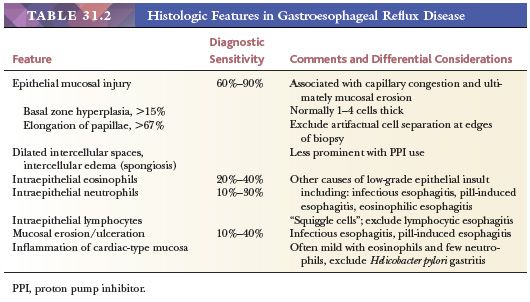
Epithelial hyperplasia is manifest by expansion of the basal zone and elongation of lamina propria papillae (Fig. 31.2). Basal zone expansion is typically specified as a basal zone exceeding 15% of the total epithelial thickness; papillary elongation is defined as lengthening greater than two-thirds of the epithelial thickness. These findings are indicative of increased epithelial proliferation and turnover and, since their description in 1970 (33), have garnered much attention as manifestations of mild reflux-induced injury (23,29,31,34,35). Most studies confirm that epithelial hyperplasia serves as a marker of reflux, but the sensitivity of this feature is debated, with reported values averaging 60% to 90% depending on cutoff and biopsy location (31). An additional issue is that accurate evaluation of epithelial hyperplasia requires well-oriented biopsies sectioned perpendicularly to the mucosal surface. Judging the basal-zone thickness may also present problems because this layer is not obviously demarcated and is prone to observer variation (34). The basal layer consists of closely packed cells with round to oval nuclei, slightly basophilic cytoplasm, and such small amounts of cytoplasm that the distance between nuclei is less than the nuclear width (36). Dilation of intercellular spaces (spongiosis), originally described as an ultrastructural finding (37), is another reproducibly identifiable early marker of GERD (37–41).
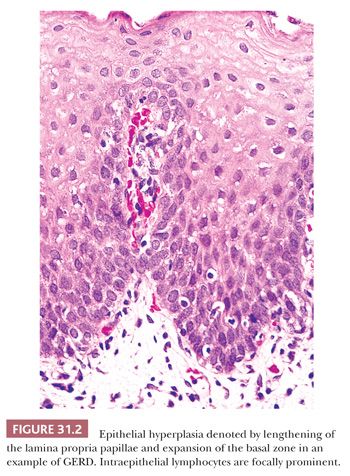
Intraepithelial eosinophils are an early indicator of GERD (Fig. 31.3) (35,42,43). Because specimen orientation is not crucial to its interpretation, this is a particularly useful feature when evaluating poorly oriented biopsies. The diagnostic value of intraepithelial eosinophils is limited by poor sensitivity; they are found in only 20% to 40% of cases of GERD, despite being the only histologic abnormality in 10% to 25% of patients (29,31,42,43). In addition, a few eosinophils may occasionally appear in the epithelium of normal asymptomatic adult subjects with normal endoscopic findings and 24-hour pH monitoring (31). Although characteristic of GERD, intraepithelial eosinophils are not specific and can be found in other circumstances, including infectious esophagitis, pill-induced esophagitis, eosinophilic esophagitis, Crohn disease, collagen vascular disease, hypereosinophilic syndrome, and eosinophilic gastroenteritis, among others (29,44,45).
Neutrophil infiltration denotes more severe degrees of epithelial injury and is consequently an insensitive diagnostic marker, being noted in only 10% to 30% of cases (31,34,46). This infiltration is associated with other features of GERD and is especially prominent in and around epithelial erosions and ulcerations of any cause. Other forms of active esophagitis, including infectious and pill-induced, thereby enter the differential diagnosis. Only present in about 40% of GERD patients, mucosal erosion and ulceration represent the extreme end of the GERD spectrum (23). Biopsy specimens show the expected fibrinopurulent inflammatory exudate, necrotic slough, and active granulation tissue, and sections should be carefully examined to exclude infectious agents or malignancy. In particular, the presence of inflammatory exudate strongly suggests mucosal destruction, even when it has not been directly sampled by the biopsy specimen, and the sections should then be studied for possible infectious organisms. The granulation tissue composing the ulcer bed can contain large, atypical mesenchymal cells that can be mistaken for carcinoma; immunohistochemical (IHC) stains for cytokeratins are helpful in excluding this possibility. Similarly, the squamous epithelium adjacent to the erosion or ulcer may demonstrate reactive atypia and prominent hyperplasia that can mimic squamous dysplasia or even invasive squamous cell carcinoma.
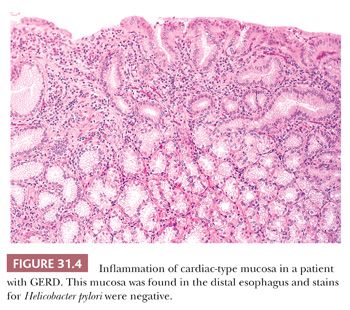
Inflammatory changes in cardiac-type mucosa (“carditis”) located near the gastroesophageal junction region (7,8,47–49) may be related to GERD or to Helicobacter pylori infection, although a minimal degree of mononuclear inflammation in this region is considered normal (5). The inflammation seen in GERD is characterized by eosinophil, neutrophil, and plasma cell infiltration of the lamina propria with variable degrees of active glandular injury (Fig. 31.4), which can be similar to H. pylori–associated carditis (47,48). Some studies have proposed that eosinophils predominate in GERD-associated carditis, whereas neutrophils and plasma cells predominate in H. pylori–associated carditis (50).
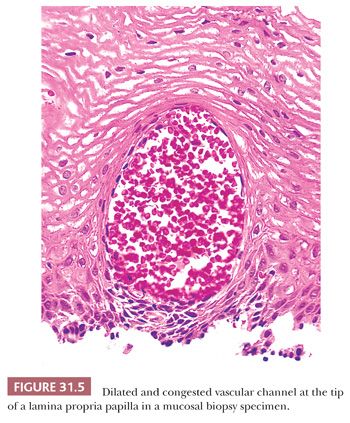
Other proposed indicators of epithelial injury include dilation and congestion of capillaries, balloon cells, and glycogenic acanthosis. Congestion or hemorrhage in the superficial papillae (Fig. 31.5) (51) corresponds to the red streaks noted at endoscopy. Unfortunately, this feature is of little use as an independent indicator because it is commonly accompanied by other markers of esophagitis; it is found in 10% to 30% of normal controls and in patients with esophageal varices; and in some cases, it probably represents an artifact of the biopsy procedure (29,34,51). Balloon cells are rounded, pale squamous cells swollen from leakage of extracellular fluid and proteins into the damaged cells (52). Balloon cells are common in patients with GERD but are also seen in active esophagitis of other causes and in patients without GERD (29,52). Glycogenic acanthosis (53), as noted in the following texts, is characterized by enlarged, glycogen-laden squamous cells, but its association with GERD has not been generally accepted.
Finally, intraepithelial lymphocytes (mostly T lymphocytes) are a normal attribute of the esophageal mucosa but can be increased in number as part of the inflammatory reaction in GERD (54,55). Patients with GERD have more lymphocytes per high-power field than normal specimens, which generally demonstrate fewer than about 10 lymphocytes per high-power field (28). The lymphocytes typically have irregular nuclear contours and appear to be entrapped between adjacent squamous cells, thereby resembling neutrophils. An increase in intraepithelial lymphocytes correlates with the presence of intraepithelial eosinophils and, thus, is neither an independent nor a sensitive histologic indicator of GERD.
The presence of a marked increase in peripapillary lymphocytes with associated spongiosis in the absence of eosinophils or neutrophils has been described as lymphocytic esophagitis (56–58). The process is patchy and may affect the mid and/or distal esophagus (58). This is a histologic diagnosis with poorly understood clinical significance. It has been described in both adults and children and may be seen in patients with Crohn disease, H. pylori gastritis, or GERD. Symptoms of dysphagia, chest pain, and heartburn often improve with PPI therapy (57). Other symptoms may include odynophagia or esophageal dysmotility, and endoscopy may be unremarkable or reveal features similar to those seen in eosinophilic esophagitis, described in the following section (58).
EOSINOPHILIC ESOPHAGITIS
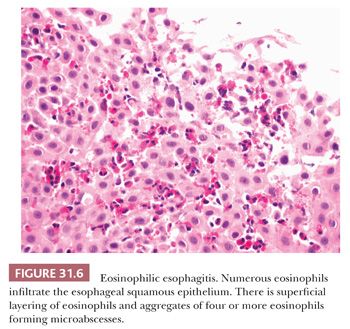
An entity in the histologic differential for GERD is eosinophilic esophagitis, an allergic disorder occurring predominantly in children, but also in adults (59). Feeding difficulties in infants and toddlers, nausea and vomiting in older children, and dysphagia in adults are common presenting symptoms, and many patients have a history of other allergic disorders (60,61). Endoscopic findings include esophageal rings, which may be fixed (“trachealization”) or transient (“felinization”), as well as longitudinal furrows, mucosal plaques, and esophageal narrowing (59). Mucosal biopsies from the proximal and distal esophagus, as well as from any endoscopically visible lesions, should be submitted in separate jars. Histologic features encountered in eosinophilic esophagitis include greater than or equal to 15 intraepithelial eosinophils in a high-power field of both proximal and distal mucosa as well as eosinophil microabscess formation (a cluster of at least four eosinophils), superficial layering of eosinophils (Fig. 31.6), eosinophil degranulation, and architectural changes including intercellular edema (spongiosis), hyperplasia of the basal zone, and elongation of papillae (62). Ultimately, the final diagnosis relies on clinicopathologic correlation, as none of the clinical symptoms, endoscopic findings, or histologic features in isolation are specific to eosinophilic esophagitis. It is especially important to note that GERD is not defined as having fewer than 15 intraepithelial eosinophils in a high-power field, as more than 15 eosinophils may be found (63).
INFECTIOUS ESOPHAGITIS
The esophagus is host to few infections. Numerous organisms may involve the esophagus as part of a disseminated illness; in practice, however, most infectious esophagitis is caused by Candida species, herpes simplex virus, or cytomegalovirus. Affected patients often have some reason for impaired host response: immunosuppression (caused by congenital conditions, infection with human immunodeficiency virus, or cytotoxic and immunosuppressive therapy), malignant neoplasms, chronic debilitating disease, diabetes mellitus, or antibiotic therapy (64,65). Normal hosts, however, are not exempt from infectious esophagitis (66,67). Because effective therapy is often available, careful scrutiny for infectious agents is indicated, especially when biopsies show ulceration or exudate. The possibility of multiple infections should also be kept in mind. Multiple biopsies may be required to identify the causative organism (68). By sampling wide areas of the esophagus, brush cytology can be an effective adjunct in identifying the causative organisms (69).
Candida Esophagitis
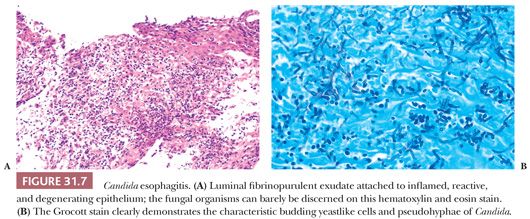
Candida species are the best known cause of infectious esophagitis. The usual culprit is Candida albicans, although sometimes Candida tropicalis, Candida krusei, or Candida glabrata are also implicated. The gross appearance at endoscopy classically entails discrete or confluent white plaques issuing from erythematous, edematous, ulcerated, or diffusely friable mucosa of the mid or distal esophagus (70). These plaques are composed of the diagnostic fungal pseudohyphae and budding spores embedded in keratinous squamous material, fibrinous exudate, and necrotic debris (Fig. 31.7A). Special stains for fungi such as periodic acid-Schiff (PAS) or methenamine silver facilitate detection of the organism (Fig. 31.7B). The plaques overshadow the underlying epithelial changes of active esophagitis with variable neutrophilic inflammation, mucosal erosion, or ulceration with inflamed granulation tissue. Because Candida is a ubiquitous commensal of the gastrointestinal tract, fungal invasion into tissue or ulcer slough is required for a definite diagnosis. In rare cases, the organism can invade deeply into the esophageal wall and result in perforation and disseminated candidiasis. Chronic infection with this organism has been associated with esophageal strictures and intramural pseudodiverticulosis (71,72).
Herpes Esophagitis
Esophagitis due to herpes simplex virus is usually seen as an opportunistic infection in immunosuppressed patients, but it also can occur in otherwise healthy children and adults (67). Grossly, the mucosa exhibits small vesicles that evolve into discrete shallow ulcers; in severe cases, these coalesce into extensive zones of mucosal denudation (73). The ulcer bed is histologically undistinguished, consisting of necrotic debris and neutrophil-rich inflammatory exudate. The diagnostic herpetic inclusions are located in discohesive or multinucleated squamous cells at the margins of the ulcers (Fig. 31.8A). The characteristic inclusions include dense, eosinophilic, intranuclear (Cowdry type A) bodies, which are separated from a thickened nuclear membrane by a clear halo, and homogeneous ground-glass inclusions that fill the nuclei. Herpetic ulcers may be secondarily infected by fungi or bacteria. In healthy hosts, herpes simplex esophagitis is usually self-limited, but in patients with an underlying predisposing condition, the result may be esophageal perforation or disseminated infection.
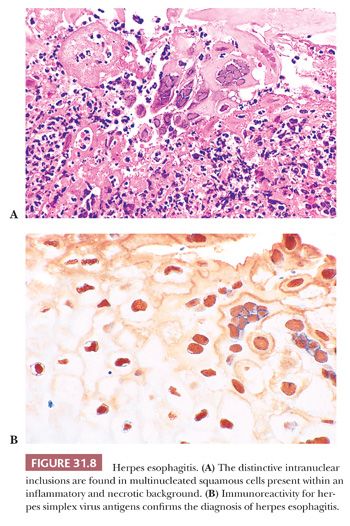
Documenting herpes simplex esophagitis can be difficult. Biopsy sampling may miss the ulcer edges where the diagnostic changes reside, or these changes may be obscured by necrosis and inflammation. The presence of ulceration in biopsies from susceptible patients should always raise suspicion, especially when prominent aggregates of large mononuclear cells are noted in the exudate (74). IHC methods can be helpful in confirming the presence of herpesvirus antigens in suspect or questionable cases (Fig. 31.8B) and may demonstrate infected cells before cytopathic effects are histologically recognizable.
Cytomegalovirus Esophagitis
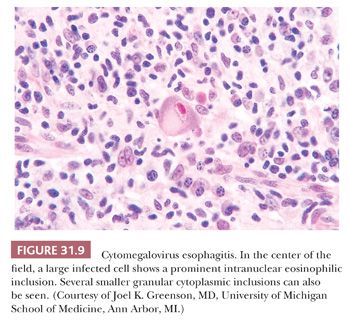
Cytomegalovirus (CMV) can infect the esophagus of immunocompromised patients (and only rarely normal individuals [66]), producing ulcers similar to those of herpes simplex esophagitis (75). The ulcer bed is the diagnostic focus: enlarged mesenchymal cells of the granulation tissue contain the heralded CMV inclusions—conspicuous intranuclear bodies surrounded by a clear halo together with, in many infected cells, coarse intracytoplasmic granules (Fig. 31.9). In patients under antiviral therapy, however, the inclusions may be less distinctive and the appearances mistaken for enlarged and reactive, but noninfected, cells. The presence of macrophage aggregates within the granulation tissue, particularly in a perivascular distribution, may be a diagnostic clue to CMV esophagitis (76). Immunostains are valuable in establishing the diagnosis when definitive inclusions are not seen.
Other Infections
Rare examples of fungal esophagitis caused by Aspergillus and the Phycomycetes are reported. In addition, histoplasmosis, blastomycosis, and cryptococcosis may involve the esophagus, usually in association with either disseminated or mediastinal disease (77–81). Unusual examples of varicella-zoster virus esophageal infections have also been reported (82).
Bacterial colonization of esophageal ulcers is a frequent observation but, in severely immunocompromised patients, bacteria can also become a primary opportunistic pathogen (83,84). Implicated bacteria include a range of Gram-positive and Gram-negative organisms, with a mixed population (chiefly representing oropharyngeal flora) commonly noted. Histologically, bacterial esophagitis is characterized by clusters of bacteria invading the esophageal wall and involving blood vessels with associated necrosis. Other unusual esophageal infections include bacillary angiomatosis (85,86), tuberculosis (87–89), actinomycosis (90), syphilis, leishmaniasis (91,92), Whipple disease (93), and Chagas disease.
Idiopathic esophageal ulcers are sometimes found in patients infected with HIV (94–96). Although HIV has been identified in these ulcers by IHC and electron microscopic studies, its presence probably does not reflect a pathogenic role, and other, as yet unidentified, agents may instead be responsible (97). The ulcers may heal with empiric therapy using corticosteroids.
MISCELLANEOUS CAUSES OF ESOPHAGITIS
Additional types of esophagitis are biopsied primarily to exclude infection and malignancy. Although histologic features may suggest the cause, the microscopic appearances are typically not specific, and the location of the lesions and the clinical data are the diagnostic attributes.
Corrosive esophagitis is caused by the ingestion of caustic agents, including strong alkalis, acids, and nonphosphate detergents, either accidentally or with suicidal intent (98,99). These agents produce varying degrees of esophageal damage, ranging from mucosal erythema and edema to extensive ulceration, hemorrhage, and necrosis with thrombosis and secondary bacterial invasion. Potential complications include stricture formation, Barrett esophagus, and squamous carcinoma, although the risk of the latter is quite low. Biopsy specimens are seldom obtained from the acute phase of injury, but they may be employed to assess complications that occasionally develop.
Pill-induced esophagitis refers to the esophageal injury resulting from direct contact of medication with mucosa. A wide range of drugs have been implicated; frequently cited agents include doxycycline and other antibiotics, aspirin and other nonsteroidal anti-inflammatory drugs (NSAIDs), slow-release potassium preparations, ferrous salts, and alendronate (100–105). The local caustic action of these agents leads to discrete erosions or ulcers without distinguishing histologic features. Aside from ulcer and granulation tissue, polarizable foreign material and multinucleated giant cells are often seen. This injury is commonly located in the midesophagus, the site of anatomic narrowing produced by the aortic arch or by an enlarged left atrium. Another cause of iatrogenic esophageal ulceration is the injection of sclerosing agents for treatment of esophageal varices. Varying degrees of localized necrosis and vascular thrombosis leading to ulceration and fibrosis are described, although biopsy specimens are seldom obtained (106). Other drugs can produce active esophageal injury through mechanisms other than direct mucosal contact. The best examples are cytotoxic chemotherapeutic agents, which can cause an active esophagitis with features ranging from mild inflammation to erosions, ulcerations, and strictures. Care must be taken in this setting to exclude infectious causes.
Sloughing esophagitis is thought to be an uncommon type of pill-induced esophagitis occurring in elderly debilitated patients taking five or more medications, often including central nervous system depressants or medications known to cause esophageal injury (107). This is a clinicopathologic entity with endoscopically visible white plaques or membranes with corresponding superficial necrosis of squamous epithelium, parakeratosis, and variable acute and chronic inflammation, without evidence of infectious cause (107,108). In patients with extended survival despite their comorbid conditions, endoscopic and histologic resolution can occur (107,108).
Radiation esophagitis develops as a complication of radiation therapy directed at neoplasms of the lung, the mediastinum, or the esophagus itself (109). The risk of radiation-induced injury increases at higher radiation doses and can be potentiated by concurrent administration of cytotoxic chemotherapy (110,111). Acute radiation damage, which occurs during the first few weeks after treatment, is manifest by mucosal necrosis and submucosal edema but is seldom biopsied. Chronic radiation injury develops weeks to months after irradiation, usually when fractionated doses exceed about 60 Gy (112). The histologic changes are characterized by progressive submucosal fibrosis, capillary telangiectasia, thick-walled hyalinized arterioles, and atrophy of mucous glands, leading to persistent ulceration, strictures, or fistulae (113). Although mucosal biopsies usually show only nonspecific changes of active esophagitis, granulation tissue, and fibrosis, the presence of atypical fibroblasts and “smudged” hyalinized collagen may suggest the correct diagnosis.
Crohn disease rarely affects the esophagus. The pathologic features mirror those found elsewhere in the gastrointestinal tract: discrete ulcers, granulomas, fistulae, and transmural inflammation (114). The disease is usually associated with disease elsewhere in the gastrointestinal tract (115), although it can present with isolated esophageal involvement (116). A definitive diagnosis requires a resected specimen, but aphthous ulcers and granulomas on mucosal biopsy may be suggestive in the proper setting.
Chronic graft-versus-host disease may be accompanied by a necrotizing and desquamative esophagitis of the proximal esophagus (117–119). Apoptosis of individual squamous cells in an uninflamed epithelium is the diagnostic finding, just as in cutaneous graft-versus-host disease. Diverse skin disorders, including lichen planus (120), pemphigus (121), bullous pemphigoid, epidermolysis bullosa, and Behçet syndrome (122), can also involve the esophagus.
Rarely, examples of severe necrotizing esophagitis or esophageal ulceration are recognized that lack a clearly defined cause despite extensive diagnostic evaluation (123).
BARRETT ESOPHAGUS (COLUMNAR EPITHELIUM-LINED ESOPHAGUS)
Barrett esophagus is a complication of chronic gastroesophageal reflux and results in the replacement of the normal stratified squamous epithelium of the esophagus with columnar epithelium of various types (124). A variety of risk factors for the development of this disease have been implicated including male gender, Caucasian race, advanced age, obesity, the presence of a hiatal hernia, history of smoking, duodenogastric reflux, delayed esophageal acid clearance times, and decreased resting pressure of the lower esophageal sphincter (125–129). Diagnosis of Barrett esophagus is important because of the significantly increased risk for esophageal adenocarcinoma (130–134).
The general concept is that destruction of the normal squamous epithelium produced by chronic reflux is followed by reepithelialization by a columnar epithelium that is more resistant to the damaging effects of acid, pepsin, and bile. Although considered an irreversible condition, regression of Barrett esophagus can be induced by acid suppression and ablative therapies, although relapse may occur (135–138).
Barrett esophagus is noted in up to 14% of patients with symptomatic GERD who undergo endoscopy with biopsy (125,139). Although the prevalence varies with the population under study, it is estimated to affect 1.6% of individuals in the general population (139). Its prevalence increases with age; thus, it is primarily a disorder of adults, although cases in children are also clearly recognized (129,140–143). Men are affected about twice as often as women.
The definition of Barrett esophagus has been refined over the past 20 years. In studies published in the 1970s and 1980s, Barrett esophagus was commonly defined by the presence of at least 2 to 3 cm of columnar epithelium in the lower esophagus. However, more recent studies have found that only those patients with intestinal metaplasia (defined by the presence of acid mucin–containing goblet cells) are at increased risk of developing dysplasia and adenocarcinoma. Accordingly, the definition of Barrett esophagus has now become dependent upon the identification of goblet cells, regardless of the length of columnar-lined esophagus. The American College of Gastroenterology and its Practice Parameters Committee define Barrett esophagus as “a change in the esophageal epithelium of any length that can be recognized as columnar type mucosa at endoscopy and is confirmed to have intestinal metaplasia by biopsy of the tubular esophagus” (144). Thus, the presence of goblet cells has become the accepted diagnostic criterion of this disease, regardless of the precise site of the biopsy within the tubular esophagus.
THE ENDOSCOPIC AND HISTOLOGIC DIAGNOSIS OF BARRETT ESOPHAGUS
Endoscopically, many patients with Barrett esophagus have a hiatal hernia, which makes identification of the gastroesophageal junction more difficult. Although the gastroesophageal junction appears to be closely approximated by the most proximal margin of the gastric folds, precise endoscopic localization of the gastroesophageal junction may be difficult in some cases. However, if it is clear that a biopsy specimen has been procured from an endoscopically identified lesion in the tubular esophagus, then the identification of goblet cells is diagnostic of Barrett esophagus.
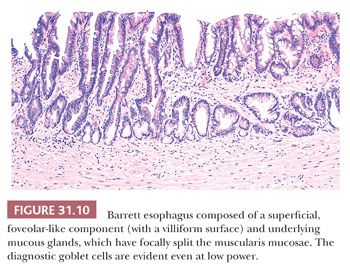
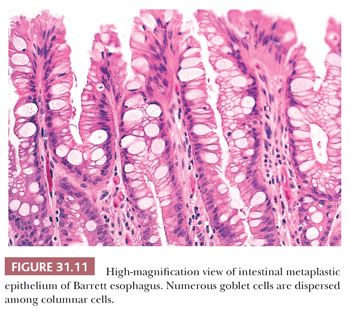
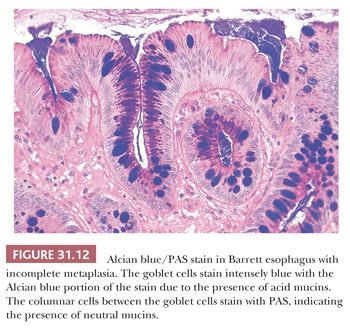
Specialized columnar epithelium is characterized by the presence of goblet cells (intestinal metaplasia), which have distended, mucin-filled cytoplasm and a barrel-shaped configuration (Figs. 31.10 and 31.11) (145). Histochemically, goblet cells contain both sialomucins and sulfated mucins that stain positively with Alcian blue at pH 2.5 (146) (Fig. 31.12). The presence of intestinal metaplasia distinguishes Barrett esophagus from several other types of glandular epithelium that may occur in the esophagus (Table 31.3). Goblet cells are not distributed uniformly in Barrett esophagus; their proportion varies considerably among patients and specimens (147). The columnar cells in between the goblet cells may resemble either gastric foveolar cells or intestinal absorptive cells (148). Pseudogoblet cells, which have a similar shape as goblet cells but contain neutral mucins (eosinophilic on hematoxylin and eosin stain), may be a diagnostic pitfall (Fig. 31.13). The predominant form of intestinal metaplasia in Barrett esophagus—incomplete intestinal metaplasia—is composed of an admixture of goblet cells and foveolar-type cells that contain PAS-positive neutral mucins. These foveolar-type cells may also contain Alcian blue–positive acid mucins, although the staining intensity is less than that seen in the goblet cells, and the identification of these Alcian blue–positive columnar cells (so-called columnar blue cells) in the absence of goblet cells is insufficient for a definitive diagnosis of Barrett esophagus (149,150). Less commonly, the glandular component contains a variable number of goblet cells, well-developed intestinal absorptive cells, and sometimes even neuroendocrine cells and Paneth cells (complete intestinal metaplasia). In some cases, there is an admixture of incomplete and complete intestinal metaplasia. Pancreatic acinar metaplasia may also be found but is of little diagnostic significance (151). Typically, the lamina propria demonstrates mild chronic inflammation and fibrosis, and the muscularis mucosae is often markedly thickened, splayed, or duplicated.
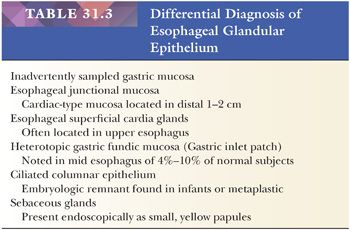
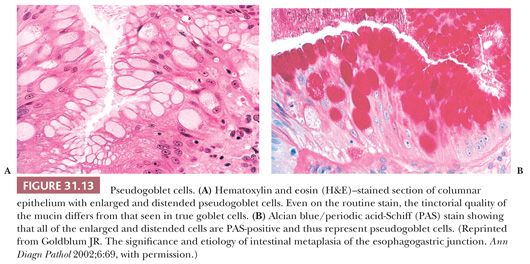
Intestinal metaplasia is variably admixed with foci of cardiac-type or fundic-type epithelium, which to some degree resemble their normal counterparts in the stomach except for the presence of mucosal distortion, glandular atrophy, and mild inflammation (152) (Fig. 31.14). A biopsy specimen from the distal esophagus with either cardiac-type or fundic-type mucosa is not diagnostic of Barrett esophagus in the absence of intestinal metaplasia. However, if the endoscopic impression is clearly that of Barrett esophagus (i.e., at least 2 cm of columnar-lined esophagus), then the absence of goblet cells may simply be a function of sampling error (153).
INTESTINAL METAPLASIA OF THE GASTROESOPHAGEAL JUNCTION
Intestinal metaplasia may be found in biopsy specimens taken near the gastroesophageal junction in 9% to 36% of patients without an endoscopically recognizable segment of Barrett mucosa (154–157). However, given the difficulty in precisely localizing the gastroesophageal junction by endoscopic techniques, it is not clear whether the intestinal metaplasia identified in these patients comes from just above or just below the gastroesophageal junction, and intestinal metaplasia of the upper stomach and distal esophagus may be histologically indistinguishable. Incidental goblet cells seen in biopsies from the junction in the absence of an endoscopic lesion do not fulfill the criteria for a diagnosis of Barrett esophagus and are of questionable clinical significance (158). However, Leodolter et al. (159) reported that more than 20% of patients with GERD and intestinal metaplasia of the cardia without endoscopic suspicion of Barrett esophagus progressed to Barrett esophagus within 2 to 5 years. Although the data are controversial, several studies support H. pylori as an etiologic factor for intestinal metaplasia of the proximal stomach (cardia intestinal metaplasia) (47,48,160,161). Intestinal metaplasia is often found in patients with small junctional adenocarcinomas, sometimes in the absence of Barrett esophagus (162). In addition, although prospective data are relatively sparse, it has been suggested that the risk of progression to dysplasia and adenocarcinoma for cardia intestinal metaplasia is significantly lower than for Barrett esophagus (163–168). If future studies continue to bear out a significant clinical difference between short-segment Barrett esophagus and cardia intestinal metaplasia, then communication of the biopsy site and presence of an endoscopic lesion will remain critical for the accurate diagnosis of Barrett esophagus.
DYSPLASIA IN BARRETT ESOPHAGUS
Barrett esophagus would remain an intriguing pathologic novelty except that it represents a premalignant condition predisposing to esophageal adenocarcinoma. The magnitude of this risk is not clearly established, but the incidence ranges from 4.1/1000 patient-years in patients with no dysplasia to 65.8/1000 patient-years in patients with high-grade dysplasia (169,170). Although all patients with Barrett esophagus are at increased risk, certain patients are at higher risk than others. As previously mentioned, there is evidence to support the contention that only those patients with goblet cells are at increased risk of developing adenocarcinoma (171–173). The presence of epithelial dysplasia, particularly high-grade dysplasia, is also a risk factor for synchronous or metachronous adenocarcinoma (132,174–177). Retrospective mapping studies have noted the frequent association of intestinal metaplasia and dysplasia to Barrett-related adenocarcinomas (171). Prospective studies have also documented a progression from intestinal metaplasia to dysplasia and eventually invasive adenocarcinoma (170,174). Although adenocarcinoma can arise in very short segments of Barrett esophagus (178), the risk of cancer is increased as the length of Barrett esophagus increases (179–183). Additional risk factors for the development of Barrett esophagus–associated dysplasia and adenocarcinoma are male gender, duration of Barrett esophagus greater than 10 years, and presence of esophagitis (124,170,183). Endoscopically, dysplasia may appear as a nodule, erosion, or polyp, but not uncommonly, dysplasia may also occur in mucosa that appears endoscopically normal, emphasizing the need for thorough sampling. In most institutions, patients are placed into a cancer surveillance program once a diagnosis of Barrett esophagus has been established, the goal of which is to identify epithelial dysplasia in a biopsy specimen before carcinoma has developed. Dysplasia surveillance includes four-quadrant biopsies taken every 1 to 2 cm throughout the length of the Barrett segment with additional biopsies of any endoscopic lesions (144).
THE PATHOLOGIC DIAGNOSIS OF BARRETT ESOPHAGUS–RELATED DYSPLASIA
Dysplasia can be defined as the presence of neoplastic epithelium confined within the basement membrane of the gland from which it arises (184). It is characterized by a combination of cytologic and architectural alterations and classified as either low-grade or high-grade based on the degree of the abnormality present. Possible diagnoses include negative for dysplasia, low-grade or high-grade dysplasia, or changes indefinite for dysplasia. There are two distinct subtypes of dysplasia arising in Barrett esophagus: the well-established adenomatous or intestinal type and the gastric foveolar type, the characteristics of which have only recently been described (185–188). Adenomatous-type dysplasia will be discussed first.
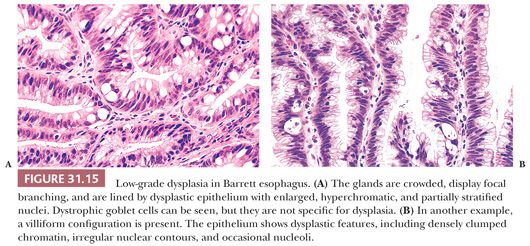
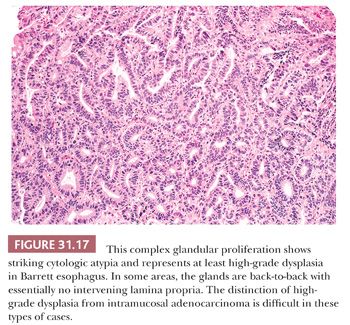
Low-grade dysplasia shows preservation or only minimal distortion of crypt architecture and is characterized cytologically by closely packed, overlapping, elongated basal nuclei with variable hyperchromasia, and irregular nuclear contours (145,189,190) (Fig. 31.15). Goblet cell numbers are often reduced, and dystrophic goblet cells may be present. High-grade dysplasia shows more severe cytologic atypia and architectural complexity, although the distinction between low-grade and high-grade dysplasia may be somewhat arbitrary in some cases. High-grade dysplasia usually has loss of cell polarity and enlarged rounded nuclei with prominent mitotic figures, including atypical mitotic figures (Fig. 31.16). Apoptosis may also be increased. There is also more crypt complexity with branched, cribriform glands and a back-to-back pattern of closely packed glands. In some cases, the distinction between high-grade dysplasia and intramucosal adenocarcinoma (defined by the penetration of neoplastic cells through the basement membrane to infiltrate into the lamina propria or muscularis mucosae) may be difficult, particularly in a biopsy specimen (191,192) (Fig. 31.17). Biopsy diagnoses of “high-grade dysplasia with marked glandular distortion, cannot exclude intramucosal carcinoma” or “high-grade dysplasia suspicious for adenocarcinoma” are associated with a significantly higher likelihood of finding carcinoma in esophagectomy specimens than a diagnosis of high-grade dysplasia alone and include any of the following histologic features: “never-ending” glands, sheetlike growth, angulated glands, at least three glands with intraluminal debris, or more than one focus of single-cell infiltration into the lamina propria (193,194).
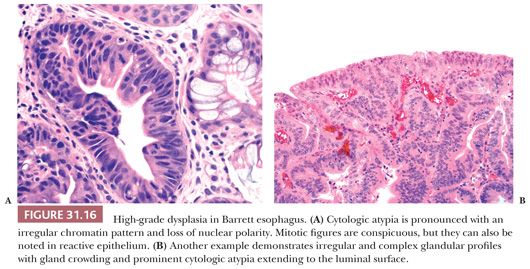
Because of its metaplastic nature, the glands at the base of Barrett mucosa show “baseline atypia” characterized by enlarged, slightly hyperchromatic cells with some stratification and increased mitotic activity. Thus, cytologic atypia involving the surface epithelium is a major diagnostic criterion for making a definitive diagnosis of dysplasia. That said, in certain cases, a diagnosis of dysplasia is prudent in cases with no definite surface involvement (195), but one should be very cautious when diagnosing dysplasia in this setting. Many patients with Barrett esophagus have ongoing GERD and active inflammation resulting in regenerative cytologic changes that may be difficult to distinguish from dysplasia. Thus, one should be cautious in making a diagnosis of dysplasia in the face of active inflammation. Cytologically, dysplastic epithelium shows variable nuclear hyperchromasia and nuclear pleomorphism. Although these features may also be seen in reparative nuclei, the changes tend to be less severe and more uniform, with cells resembling their neighbors within the same gland or in adjacent glands. Discrimination between the two processes is not always straightforward. In such cases, a diagnosis of “indefinite for dysplasia” is appropriate, signifying that the histologic changes are neither unequivocally neoplastic (“positive for dysplasia”) nor clearly reactive (“negative for dysplasia”). The “indefinite” category is often employed when epithelial atypia is noted in a background of active neutrophilic inflammation and/or mucosal erosion; because the abnormalities could possibly represent florid regeneration, an express diagnosis of dysplasia is unsuitable. Obtaining additional biopsy specimens after successful antireflux treatment is often helpful in resolving these problems. A diagnosis of indefinite may also be employed when the architectural or cytologic features are obscured by fixation, sectioning, or staining artifacts, or when the findings fall quantitatively or qualitatively short of a definitive diagnosis of dysplasia.
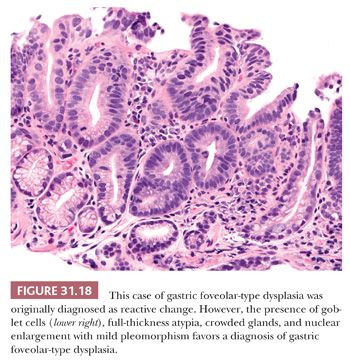
Gastric foveolar-type dysplasia has basally oriented nuclei, which do not show stratification or loss of nuclear polarity (186). High-grade dysplasia is therefore identified by the presence of enlarged nuclei (three to four times the size of a lymphocyte), mild nuclear pleomorphism, prominent nucleoli, presence of eosinophilic cytoplasm, crowded and irregular glandular architecture, and villiform architecture (186). Low-grade dysplasia has monomorphic basally oriented nuclei, nuclear enlargement (two to three times the size of a lymphocyte), prominent nucleoli, foveolar mucinous cytoplasm, and increased number of glands occupying the full thickness of the mucosa (186). IHC staining for foveolar-type and intestinal-type mucins (i.e., MUC5AC and MUC2, respectively) may be helpful in distinguishing gastric foveolar-type dysplasia from intestinal-type dysplasia (187). Features helpful to distinguish gastric foveolar-type dysplasia from reactive epithelial changes associated with GERD include the presence of villiform architecture, full-thickness atypia, and crowded glands in dysplasia and the presence of surface nuclear stratification and atypia limited to the upper mucosa in GERD (188) (Fig. 31.18). Also, because gastric foveolar-type dysplasia arises in Barrett’s esophagus, goblet cells will be present, although they may be sparse.
Both sampling error and observer variation in the diagnosis of dysplasia make management of these patients difficult (196). Dysplasia can be diffusely distributed throughout a Barrett segment but, in some cases, the dysplastic alterations are extremely focal (197). Thus, even when using a rigorous endoscopic sampling technique, small dysplastic foci can be left unsampled. Similarly, 12.7% to 17.6% of patients with a preoperative diagnosis of high-grade dysplasia are found to have adenocarcinoma in the esophagectomy specimen (198,199), which is still significant, although less than the 40% reported in earlier literature (200). Finally, there is significant interobserver variation in the diagnosis of Barrett-related dysplasia, particularly in the separation of “negative” versus “indefinite” versus “low-grade” dysplasia (189,201–205). This emphasizes the need to obtain multiple opinions on difficult cases.
DYSPLASIA
NATURAL HISTORY OF DYSPLASIA
Although it is clear that high-grade dysplasia is the precursor lesion to esophageal adenocarcinoma, it is not clear how often or how rapidly this lesion progresses. A meta-analysis reported the incidence of esophageal adenocarcinoma in patients with high-grade dysplasia to be approximately 6 per 100 patient-years during the first few years of surveillance (205). In one prospective study of patients with unifocal high-grade dysplasia with long-term follow-up by Weston et al. (165), 53% of patients progressed to either multifocal high-grade dysplasia or invasive carcinoma within the follow-up period. In contrast, a large study of patients with Barrett-related high-grade dysplasia suggested that surveillance endoscopy with biopsy is a valid and safe follow-up strategy for patients with high-grade dysplasia without concurrent cancer because only 16% of patients subsequently developed carcinoma during a mean surveillance period of 7.3 years (206). There is also controversy regarding the optimal surveillance protocol in patients diagnosed with high-grade dysplasia (207).
Even less is known about the natural history of low-grade dysplasia, primarily due to the high degree of interobserver variability that is known to exist in establishing this diagnosis (189,201). However, in cases in which there is uniform agreement of a diagnosis of low-grade dysplasia, it is clear that there is an increased risk of progression to high-grade dysplasia or an invasive adenocarcinoma (208–210). Due to earlier stage at detection associated with surveillance, patients are less likely to require esophagectomy and less likely to have lymph node metastases, contributing to improved survival (211).
SURROGATE BIOMARKERS
Numerous adjunctive techniques have been proposed as possibly having a role in the screening or surveillance of patients with Barrett esophagus, given the limitations of light microscopy described earlier. For virtually every marker studied, there is an increased frequency of abnormality of that marker with progression along the dysplasia–carcinoma sequence. However, the ideal marker would be present early in the metaplasia–dysplasia–carcinoma sequence and only in those patients who progress. As such, DNA content, as measured by flow cytometric methods, and evaluation of p53 abnormalities have shown the greatest promise as predictive markers of progression (173,212–216). For example, in a prospective study by Reid et al. (215), patients with negative, indefinite, or low-grade dysplasia biopsy specimens with neither aneuploidy nor increased 4N fraction had a 0% 5-year cumulative cancer incidence compared with 28% for those with either aneuploidy or increased 4N. Antiphosphorylated histone H3 expression (217), microRNAs (218), loss of heterozygosity (219), p16, cyclin D1, proliferation markers, and promoter methylation have also been studied (220). Immunohistochemistry for p53 is used clinically by some pathologists to assess Barrett-related neoplastic risk; however, this is not a widely accepted routine practice, as it is unclear whether this adds value beyond hematoxylin and eosin stain alone.
NEUROMUSCULAR DISORDERS
Assorted disorders of the esophagus are manifest by abnormalities in motility and sphincter function and are diagnosed by clinical, radiographic, and manometric findings. Pathologic correlates have generally been studied only in end-stage autopsy and rarely resection specimens; consequently, the nature and evolution of the early changes are relatively inaccessible and poorly understood. The surgical pathologist is therefore largely limited to evaluating the complications of disordered motility, notably reflux-associated esophagitis, Barrett esophagus, and carcinoma. Aside from achalasia, the morphologic features of these conditions are only briefly sketched.
Idiopathic muscular hypertrophy is characterized by marked thickening of the muscularis propria, particularly the inner circular layer of the distal esophagus (221,222). This is the pathologic counterpart of diffuse esophageal spasm, a rare condition of older men manifesting with dysphagia and angina-like chest pain. Functional evidence suggests that the primary abnormality is in the neural control of muscular contraction, but a neuropathologic lesion is not well documented; however, lymphocytic infiltration of the myenteric plexus is sometimes noted.
This condition should be differentiated from leiomyomatosis, in which multiple confluent nodules of disorganized smooth muscle expand the lower esophageal and proximal gastric musculature (223). Leiomyomatosis primarily affects female adolescents and young adults and may be associated with vulvar leiomyomas (224,225) and Alport syndrome (226). Interestingly, this disease has been found in patients with rectal muscular hypertrophy causing chronic constipation, thereby mimicking Hirschsprung disease (227).
Systemic sclerosis (scleroderma) commonly involves the esophagus, resulting in atrophy and fibrous replacement of the smooth muscle of the muscularis propria, chiefly the inner circular layer (228). Varying degrees of submucosal fibrosis, nonspecific inflammatory infiltration, and proliferative changes of smaller arteries may also be noted. These changes can lead to gastroesophageal reflux with the subsequent development of esophagitis, Barrett esophagus, and an increased risk of esophageal adenocarcinoma. Atrophy and fibrosis of the muscularis propria can also be seen in other conditions, including systemic lupus erythematosus, Sjögren syndrome, primary visceral myopathies, and cricopharyngeal dysphagia.
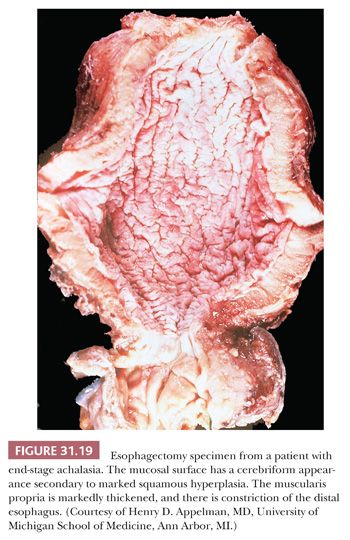
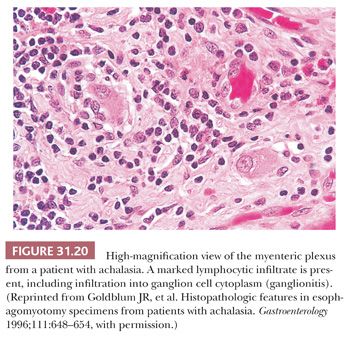
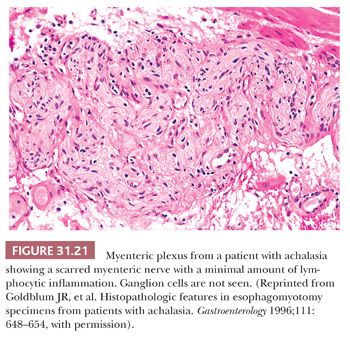
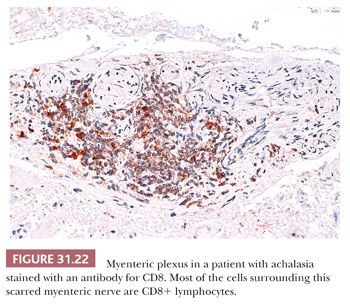
Achalasia is an esophageal motor disorder characterized by failure of relaxation of the lower esophageal sphincter and lack of progressive peristalsis in the esophageal body. This condition may occur secondary to a variety of conditions, including Chagas disease, sarcoidosis, or a host of intrathoracic malignancies or inflammatory conditions (secondary achalasia or “pseudoachalasia”) (229–231). However, most cases are idiopathic and not associated with an underlying condition (primary achalasia). The cardinal morphologic alteration is the depletion or complete absence of myenteric ganglion cells associated with a myenteric inflammatory infiltrate composed predominantly of cytotoxic T cells (232–235) (Figs. 31.19 through 31.22). These changes may be seen not only in esophagectomy specimens but also in biopsies of the muscularis propria taken at the time of myotomy. There are also a number of secondary changes that can be identified in esophagectomy specimens resected for end-stage achalasia that involve all layers of the esophageal wall (232,235,236). Patients with achalasia have a significantly increased risk of esophageal squamous cell carcinoma (237–239), which is presumably secondary to a multistep process beginning with marked squamous hyperplasia to squamous dysplasia and carcinoma similar to that seen in sporadic esophageal squamous cell carcinoma (241). Treatment of achalasia by esophagomyotomy decreases the lower esophageal sphincter muscle tone, which is often combined with an antireflux fundoplication to reduce the risk of GERD. Other complications may include peptic ulceration, fibrous stricture, and, in some cases, Barrett esophagus and adenocarcinoma (241).
BENIGN TUMORS AND TUMORLIKE LESIONS
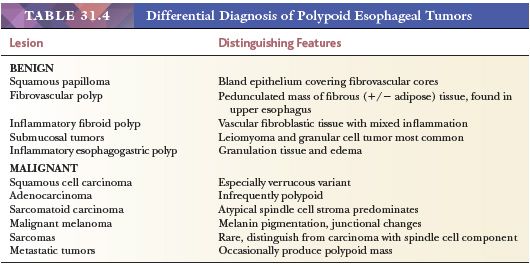
A diversity of benign tumors and nonneoplastic masses can be seen in the esophagus. They are, however, mostly uncommon lesions, small, and asymptomatic, whose importance lies in their distinction from malignant tumors. Most clinically apparent tumors grow or protrude into the lumen and thus appear as polyps at endoscopy. The differential diagnosis of polypoid esophageal lesions is summarized in Table 31.4.
SQUAMOUS PAPILLOMA
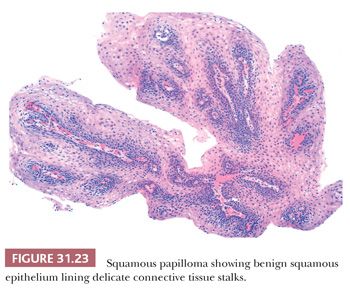
Squamous papillomas are the most common benign esophageal epithelial neoplasm (242,243). Most are small (<0.5 cm) sessile lesions, usually solitary, composed of mature acanthotic squamous epithelium arranged along branched fibrovascular stalks (Fig. 31.23) with an exophytic, endophytic, or spiked architectural pattern (242). They are rare lesions, noted in less than 0.04% of endoscopic examinations, and typically represent an incidental finding in asymptomatic patients, although larger lesions may cause dysphagia. Most arise in the distal esophagus, but they may rarely develop in the proximal or midesophagus. In some cases, condylomatous features such as koilocytosis, parakeratosis, hypergranulosis, and binucleated cells are noted, suggesting human papillomavirus (HPV) infection. Indeed, several studies have identified HPV DNA in some squamous papillomas, particularly HPV types 6, 11, 16, and 18 (242,243), but other studies have not found this association (244), suggesting that some develop secondary to chronic mucosal irritation. On occasion, squamous papillomas may be difficult to distinguish from pseudoepitheliomatous hyperplasia occurring next to an ulcer, but the latter lacks the fibrovascular cores seen in squamous papillomas. Verrucous carcinoma may also enter in the differential diagnosis but has a different endoscopic and gross appearance. There are rare reports of squamous papilloma associated with squamous dysplasia or squamous cell carcinoma (245).
ADENOMA
Rare esophageal tumors are reported that fall under the general heading of benign glandular neoplasms, but the pathologic details in these reports are often sparse. These lesions include mucous gland adenomas similar to those of the bronchus, salivary gland–like pleomorphic adenomas, parathyroid and thyroid adenomas, serous cystadenomas, and papillotubular adenomas apparently originating in the ducts of submucosal glands (246–248). Lesions with the same dysplastic histology as adenomas elsewhere in the gastrointestinal tract can also develop in Barrett esophagus, but these are best considered polypoid examples of dysplastic Barrett mucosa. These polypoid dysplastic lesions have been shown to harbor similar molecular abnormalities as those found in Barrett esophagus–related flat dysplasia (249,250).
LEIOMYOMA
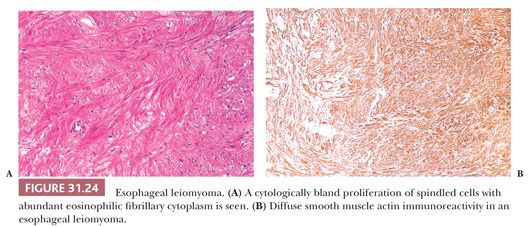
Although it is the most common benign tumor of the esophagus, leiomyoma seldom poses a clinical problem. In general, these tumors appear as circumscribed mural masses, usually solitary and 2 to 5 cm in diameter, that bulge into the lumen and may even form pedunculated polyps (251). Minute “seedling” leiomyomas of 1- to 2-mm diameter can be commonly identified in the vicinity of the gastroesophageal junction (252). The morphologic features mirror those of other classic leiomyomas: Interlacing fascicles of bland spindle cells with variable fibrosis produce the traditional circumscribed, white-gray whorled appearance on gross examination (Fig. 31.24). Distinction from the very rare esophageal leiomyosarcoma and esophageal gastrointestinal stromal tumor is not difficult based on morphologic and immunophenotypic findings (253).
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


