Esophagogastrectomy for Carcinoma of the Esophagus
Simon Y.K. Law
Surgeons who operate on the esophagus should be familiar not just with mediastinal anatomy, but also with anatomy of the upper abdomen as well as the neck, as esophagectomy and lymphadenectomy encompass all these three body compartments. The esophagus is anatomically closely related to the trachea, the left main bronchus, pericardium, aorta, and the esophageal diaphragmatic hiatus, all of which could be infiltrated. The primary tumor or metastatic lymph nodes can involve the recurrent laryngeal nerves producing hoarseness of voice. The following aspects of surgical anatomy deserve specific considerations:
The rich submucosal network of lymphatics makes longitudinal spread of tumor along the esophagus prevalent. An adequate proximal and distal resection margin must therefore be achieved. The chance of anastomotic recurrence is related to the length of proximal margin attained at operation; ideally, an in situ proximal margin of 10 cm should be sought after.
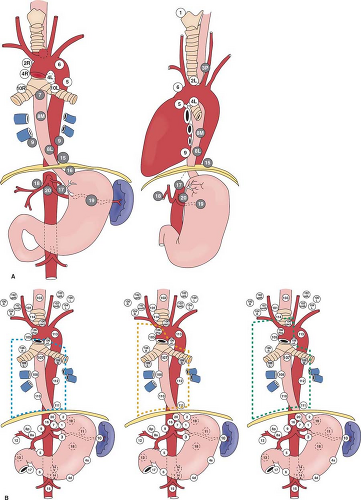
Esophageal cancer is known for its propensity of early spread, as well as widespread nodal metastases. Even for tumors that have infiltrated to the submucosa, metastatic nodes are found in 30% to 50% of patients. Metastases are commonly found in lymph nodes in the mediastinum, perigastric region and celiac trifurcation, and in the neck. Mediastinal anatomy and lymph node stations according to the American Joint Committee on Cancer (AJCC) as well as the Japan Society of Esophageal Diseases are shown in Figure 1.
For esophageal cancer of the superior mediastinal segment, nodal spread tends to be proximal. For tumors of the lower esophagus and gastroesophageal junction, it is primarily distal. Nodal spread, however, can be widespread from the cervical region to around the celiac trifurcation, especially for cancer of the mid-esophagus. Lymphadenectomy below the tracheal bifurcation and around the celiac trifurcation is relatively straightforward. The most difficult aspects of mediastinal lymphadenectomy are nodes around the recurrent laryngeal nerves; in the left paratracheal and subaortic areas for the left recurrent nerve, and below the right subclavian artery for the right recurrent nerve. Unfortunately, nodal metastases tend to be located around the recurrent nerves and their dissection risks recurrent laryngeal nerve injury.
Data mainly from Japan has shown that in relation to the level of the primary tumor, cervical lymph nodes are involved in up to 60%, 20%, and 12.5% of upper, middle, and lower third tumors, respectively. In selected centers in the United States and Europe, cervical lymphadenectomy has also demonstrated incidences of positive cervical nodes of around 30%, even when adenocarcinomas of the lower esophagus are considered.
For cervical nodal metastases, the majority of nodal metastases are located below the omohyoid muscles, especially around the paratracheal/paraesophageal area as a continuum of the recurrent laryngeal nerve nodal package from the superior mediastinum. The whole chain of nodes can be regarded as one unit.
Proponents of the concept of “En-bloc resection” advocate removing the primary tumor together with its enveloping sheath of pericardium, thoracic duct, azygos vein, intercostals vessels, and bilateral pleurae overlying the primary tumor and a surrounding cuff of crura (if the primary tumor is abutting). The concept is based on the embryologic development of the esophagus in the posterior mediastinum. This type of resection is more suitable for lower esophageal tumors only because in more proximally located tumors, resection is limited by the trachea-bronchial tree.
The tendency of the esophageal anastomosis to leak has been attributed to its lack of a strong serosa, its poor blood supply, and that its muscle fibers are orientated longitudinally. Clinical experience, however, has shown that the esophagus has an excellent blood supply, evidenced by the free bleeding from its edge when it is cut, even when a substantial length has been mobilized from its mediastinal bed. Its lack of serosa is compensated by its strong submucosa, and in any case this is irrelevant when no tension is present at the anastomosis.
The thoracic duct carries lymph from the cistern chyli in the abdomen, through the mediastinum to the neck to empty into the confluence of the internal jugular vein and brachiocephalic vein on the left side. Injury to the thoracic duct during esophagectomy risks postoperative chylothorax. Prophylactic ligation of the duct near the diaphragmatic hiatus lessens the chance of chylous leak. The thoracic duct should be resected en bloc with the esophagus to maximize lymphadenectomy.
Many of these aspects of surgical anatomy will be discussed in the following sections when surgical techniques are described.
Most patients present with symptomatic, advanced cancers. The main symptom is progressive dysphagia or sometimes bleeding, more commonly encountered with adenocarcinoma. Other symptoms include regurgitation, odynophagia, weight loss, and hemoptysis when airway involvement is present. A frank tracheobronchial fistula leads to coughing and choking on drinking and is characteristic. Hoarseness of voice indicates recurrent laryngeal nerve involvement. Bleeding from aorto-esophageal fistula is uncommon. Physical examination is usually unremarkable. The most likely site of a positive physical sign is the supraclavicular fossa, where metastatic nodes should be searched for.
Diagnosis of advanced cancers is usually straightforward by endoscopic biopsies. Diagnosing the disease at the asymptomatic or early stage requires a high index of suspicion when endoscopy is carried out, with adjuncts such as chromoendoscopy with Lugol’s iodine spray for squamous dysplasia, or using narrow-band imaging (NBI). In most countries, however, endoscopic screening is not cost-effective. Cytology and endoscopic screening is performed in certain high incidence areas in China. In western countries, Barrett’s esophagus and adenocarcinoma secondary to gastroesophageal reflux disease is prevalent. However, screening patients with symptoms of chronic reflux for Barrett’s esophagus is not universally recommended. Once Barrett’s esophagus is diagnosed, endoscopic surveillance is generally performed, the interval depending on whether dysplasia is found, and if so, its grade.
Early mucosal lesions can be resected endoscopically by endoscopic mucosal resection (EMR) or endoscopic submucosal dissection (ESD) techniques. The resected specimen can be regarded as a giant biopsy; the depth of infiltration determines the need for further treatment. Truly intramucosal cancers have a very low chance of nodal metastases and endoscopic resection can be regarded as curative. For more infiltrative lesions, further treatments include surgery, radiotherapy or chemoradiotherapy. Staging investigations for more advanced lesions should include chest radiograph, bronchoscopy (for tumors of the middle and upper esophagus), positron emission tomography scan combined with computer tomography scan (PET/CT), and endoscopic ultrasonography ± guided fine-needle-aspiration (FNA) cytology examination of suspicious nodes. Percutaneous ultrasound-guided FNA of suspicious cervical nodes can also be performed. Laparoscopy is only selectively indicated, especially for gastroesophageal junction tumors, and where noninvasive tests have yielded suspicious metastases, which when confirmed will alter treatment strategies. Thoracoscopic staging is generally regarded as too invasive.
The primary indication for surgical resection is for potential cure, which can be achieved in patients whose tumors are confined to its wall and only limited local-regional disease is found. One should aim to maximize the chance of an R0 resection (macroscopic and microscopic clearance of proximal, distal, and lateral margins), a parameter that has consistently been shown to result in the best long-term survival. Surgical resection with intentionally palliative intent is less commonly performed, although sometimes this is indicated in certain circumstances, such as a bleeding esophageal/gastroesophageal junction tumor requiring frequent blood transfusion (more commonly encountered with adenocarcinoma since squamous cell cancers rarely have significant bleeding), or a cervical esophageal cancer, which has been treated by chemotherapy or radiotherapy, but residual tumor still precludes
satisfactory oral alimentation. Situations such as these are difficult to palliate without surgical resection.
satisfactory oral alimentation. Situations such as these are difficult to palliate without surgical resection.
Increasingly, neoadjuvant treatments including chemotherapy or chemoradiotherapy are used to treat esophageal cancer. Consistently, these strategies have been shown to result in a higher rate of R0 resection, and a pathologic complete response (in resected surgical specimen) can be achieved in <10% for chemotherapy alone, and up to 30% for chemoradiotherapy. Although the benefits of neoadjuvant treatments over surgical resection alone are not proven in randomized controlled trials and remain controversial, these strategies are routinely used in many centers. In patients with more advanced staged disease where the chance of a R0 resection is less, such as c-T3/T4 disease or those with multiple local regional nodal spread, these therapies will result in significant downstaging in many patients, making a subsequent R0 resection possible. Treating patients with upfront chemoradiotherapy with curative intent is another option. However, salvage surgical resection is technically more demanding than that in the neoadjuvant setting; the radiation dose is often higher, delay to surgery is longer, and the patients’ physiologic reserve may be suboptimal having gone through prolonged courses of chemotherapy and radiotherapy. Possibility of cure, however, still exists provided only local-regional disease is present. How surgical resection should be integrated into programs of multimodality treatment remains controversial, and differs among institutions.
Patient Selection
Selecting patients for esophagectomy depends on many factors, including: (a) the referral pattern, (b) the prevailing treatment philosophy, (c) the availability of alternative therapies, and (d) the possible mortality that the surgeon and patient are prepared to accept. Reported resection rates (number of resection divided by the number of patients assessed) range from 20% to 80%; this wide variation suggests probable pre-referral bias or a high prevalence of early cancers in those with high resection rate.
Selection based on disease stage has been discussed in the above section. Other considerations mainly focus on patients’ physiologic risks. Factors often cited as being predictive of morbidity and mortality after esophagectomy include advanced age, poor performance status, nutritional depletion and weight loss, more proximally located tumor, poor pulmonary function, cirrhosis, and abnormal cardiac evaluation. Patients suffering from adenocarcinoma and squamous cell cancers also have different risk profiles. Patients with squamous cell cancers are more likely to be malnourished, have high alcohol intake, are smokers, and have more impairment of pulmonary and hepatic functions. Patients with adenocarcinomas, on the other hand, are more likely to be overweight, and are more at risk from cardiovascular diseases. Assessing a patient’s fitness is often based on surgeons’ experience and is not an exact science. Objective scores can help assess operative risk and patient selection, but scores that apply at one institution are not necessarily applicable to others.
At the author’s institution, preoperative assessments include a detailed history and clinical physical examination, simple blood profiles, chest radiograph, electrocardiogram, and pulmonary spirometry. More detailed cardiac work-up including echocardiography, myocardial perfusion scans, or angiograms is selectively applied when specific indications exist. Cirrhosis is not a contraindication to esophagectomy, although the presence of esophageal varices usually contraindicates surgery.
Preoperative Treatments
In general, limited improvement can be made to a patient’s physiologic status, perhaps with the exception of coronary revascularization with angioplasty and coronary stenting in the presence of significant coronary ischemia. Anti-thrombotic medications such as aspirin and Clopidogrel are often indicated post coronary intervention for a period of time. In such patients, it is prudent to treat them with neoadjuvant therapy, so that time is not lost in waiting for an optimal time for surgery post coronary stenting.
Patients must stop smoking and alcohol intake. Chest physiotherapy is instituted, and incentive spirometry is a good preoperative exercise. Those with chronic obstructive airway disease should have their bronchodilator therapy optimized. Patients with high-grade esophageal tumor stenosis may have lost substantial amount of weight. A fine-bore nasogastric tube can be placed for nutritional support while work-up is performed, and is preferable over parenteral nutrition or gastrostomy or jejunostomy feeding. Diabetic control should be optimized with medication or insulin therapy.
Immediate preoperative preparations include prophylactic antibiotics to be given at anesthesia induction, deep vein thrombosis prophylaxis with heparin, and anti-thrombotic stockings. Bowel preparation is not necessary, unless a colonic interposition is intended.
Choice of Surgical Approach
The choice of the appropriate technique for esophagogastrectomy depends mainly on: (a) the location of the tumor, (b) the intended extent of lymphadenectomy and reconstructive technique, and (c) the experience and preference of the surgeon. These variables are inter-related and could affect immediate morbidity and mortality rates, long-term quality-of-life, and survival. The surgeon should be versatile and well versed with the many different techniques to adapt to different clinical situations.
Tumor Location
Cervical Esophageal Cancer
In 1960, Ong and Lee first described the procedure of pharyngo-laryngo-esophagectomy (PLE) as a one-stage, three-phase operation that involved cervical and abdominal incisions and a thoracotomy. A thoracotomy is first performed for esophageal mobilization; this is followed by cervical incision for pharyngolaryngectomy and cervical esophageal extirpation. A permanent terminal tracheostomy is constructed. A laparotomy is used for delivering the gastric conduit to the neck for pharyngogastric anastomosis. Modern surgical technique has replaced the thoracotomy with transhiatal esophageal mobilization. Thoracoscopic (or laparoscopic) esophageal mobilization is also an alternative and has become our preferred method.
For tumors that are confined to the proximal portion of the cervical esophagus, with sufficient distal margin, free jejunal interposition graft and deltopectoral or pectoralis major myocutaneous flaps are options for reconstruction after resection. The use of a free jejunal graft is advantageous because it avoids mediastinal dissection, though expertise in performing microvascular anastomosis is essential. Graft necrosis, fistula formation, and late graft strictures are specific problems. When compared with gastric pull-up, graft survival and leak rates are similar. Stricture is the most common late complication for free jejunal transfers, whereas reflux is most common in gastric pull-ups, both occurring in, 20% of patients.
The need to sacrifice the larynx does make surgical resection an unattractive option, and upfront chemoradiation is used in many centers, with surgery reserved for salvage.
Intrathoracic Esophageal Cancer
For tumors in the upper thoracic esophagus, obtaining a sufficient proximal resection margin dictates an anastomosis placed in the neck. For this reason, resection is best carried out by a three-phase esophagectomy or the McKeown approach. In this procedure, a right thoracotomy is first carried out to mobilize the thoracic esophagus together with lymphadenectomy. This is followed by abdominal and neck incisions for the mobilization of the esophageal substitute placing the anastomosis in the neck.
The majority of intrathoracic cancers are squamous esophageal cancers located in the middle and lower esophagus, and Barrett’s adenocarcinomas in the lower esophagus. A common approach was that described independently by Lewis (1946) and Tanner (1947). The operation begins with an abdominal phase, in which the stomach is prepared; a right thoracotomy and resection of the tumor together with lymphadenectomy follows this. The stomach is then brought up into the chest for anastomosis with the proximal esophagus at the apex of the pleural cavity.
An alternate approach involves a single left thoracotomy incision. Through a left thoracotomy and incision in the diaphragm, both the esophagus and the stomach could be mobilized and resection carried out, and stomach delivered into the chest for anastomosis, either below or above the aortic arch. Proximally, the aortic arch does hinder surgical access, making mobilization of the proximal esophagus and subsequent anastomosis difficult. The approach is therefore more suitable for cancer of the cardia or the distal esophagus where an adequate resection margin is obtained below the aortic arch. Distally, however, exposure for the celiac trifurcation lymph node dissection and the pylorus for a drainage procedure are also hampered.
A transhiatal approach, whereby the thoracic part of the esophagus is mobilized by blunt and often blind dissection through the enlarged esophageal hiatus, and the mobilized stomach is then delivered to the neck and anastomosed to the cervical esophagus. This approach precludes thorough extended mediastinal lymphadenectomy, especially of the mid- and superior mediastinum. Randomized trials have shown lower cardiopulmonary complication rates compared to open thoracotomy; mortality rates, however, are similar. With modern surgical technique and perioperative care, however, reduction of morbidity rate by transhiatal esophagectomy should not be an overriding consideration. From a purely safety point of view, transhiatal resection is not suitable for patients with advanced middle- or upper-third tumors, especially patients with tumors closely related to the tracheobronchial tree and after neoadjuvant radiation therapy; tumor infiltration or fibrosis may obliterate tissue planes and make blind dissection unsafe. As such, its application is more suitable for distal esophageal tumors, for which much of the mobilization can be performed under visual control.
Minimally invasive esophagectomy is increasingly performed; combinations of thoracoscopy, laparoscopy, mediastinoscopy, and open laparotomy and thoracotomy have been advocated. Thoracoscopic esophagectomy enables lesser surgical trauma, and at the same time, mediastinal lymphadenectomy equivalent to that of an open approach is feasible. Laparoscopic esophagectomy (without thoracoscopy) can reproduce a transhiatal esophagectomy, and indications are similar. When minimally invasive approaches are used, a cervical anastomosis is usually performed because an intrathoracic anastomosis is more difficult with these techniques. Randomized trials comparing minimally invasive methods with open techniques are lacking, but it is evident from large published series that surgical results can be at least as good as that of open surgery, both in short-term morbidity and mortality rates, and oncologic clearance and long-term survival.
Gastroesophageal Junction Cancer
For cancers that are limited to the abdominal esophagus or gastric cardia, an abdominal-right thoracic approach as in a Lewis-Tanner esophagectomy is one option, with the proximal stomach also resected in order to gain an adequate distal resection margin. A left thoracoabdominal incision through the seventh or eighth rib space also gives excellent exposure of the low mediastinum and upper abdomen. When a thoracotomy is not desired, opening the hiatus widely by splitting the crura laterally and the diaphragm anteriorly can gain access to the low posterior mediastinum, and distal esophagectomy can be performed with the anastomosis performed from the abdomen without the need for a thoracic incision. The anastomosis is made easier with a mechanical stapler. When the proximal stomach is involved by tumor, many prefer a total gastrectomy with Roux-en-Y reconstruction. A Japanese randomized trial comparing a transabdominal versus a left thoracoabdominal approach for adenocarcinomas that have infiltrated 3 cm or less into the esophagus demonstrated no difference in long-term survival between the two. A thoracotomy can be avoided, provided frozen sections are carried out to ensure a negative proximal margin.
Intended Lymphadenectomy and Reconstructive Techniques
The relationship of the route of surgical access to the extent of lymphadenectomy has already been alluded to in the sections above; when an extended lymph node dissection is intended in the mediastinum (especially above the tracheal bifurcation), an open transthoracic or thoracoscopic approach is mandatory. When bilateral cervical nodal dissection is planned, then a cervical anastomosis makes sense.
There are other important variables to consider. An advanced esophageal tumor adjacent to the trachea, bronchi, or thoracic aorta of doubtful resectability may prompt an exploratory thoracotomy first to assess resectability, followed by the preparation of the replacement organ in the abdominal phase of the operation and a cervical anastomosis, even though the tumor may be located in the mid-esophagus, where a Lewis-Tanner esophagectomy with intrathoracic anastomosis could give adequate proximal longitudinal clearance. If radiation therapy to the posterior mediastinum after surgical resection is planned, it may be prudent to place the substitute organ in the retrosternal route to avoid radiation injury. When a colonic loop is planned for esophageal replacement, sometimes it is advantageous to prepare the colonic conduit before esophageal resection to ensure its viability, especially when the vasculature of the colon has been demonstrated to be suboptimal on preoperative arteriogram. In such cases, the abdominal phase of the operation precedes the thoracic phase, dictating a retrosternal route of conduit placement, unless a second change of body position is undertaken.
Experience of the Surgeon and Preference
Surgeon preference and experience is also an important element. A surgeon with a significant anastomotic leakage rate may decide to place the anastomoses in the neck, because a cervical leak is easier to manage and may be less lethal compared to
an intrathoracic leak. Many of the procedures described have a learning curve. No one surgical procedure fits all. An esophageal surgeon should be as versatile as possible to cope with the myriad of clinical situations.
an intrathoracic leak. Many of the procedures described have a learning curve. No one surgical procedure fits all. An esophageal surgeon should be as versatile as possible to cope with the myriad of clinical situations.
The following sections describe the three phases of an esophagogastrectomy: the abdominal, thoracic, and cervical phases. These three phases can be combined in different surgical approaches, depending on considerations detailed above. For example a Lewis-Tanner esophagectomy will involve the abdominal followed by a right thoracic phase, while a three-phase McKeown esophagectomy will involve the thoracic, followed by an abdominal and cervical phase.
Abdominal Phase
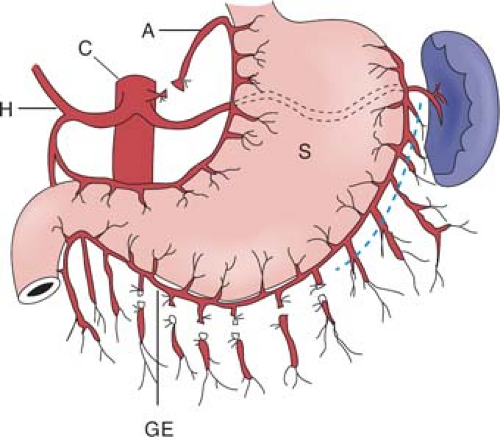
Surgical access can be gained either through an upper midline or an upper rooftop incision. The author prefers the latter because it gives improved exposure to the upper abdomen, especially in an obese patient. The stomach used for esophageal replacement is prepared by first taking the gastrocolic omentum off the greater curvature of the stomach but preserving the right gastroepiploic vessels and arcades (Fig. 2). Detachment is begun near the pyloric region and continued toward the spleen. Complete omentum resection is not necessary. Division of the gastrocolic omentum can be carried out just outside the right gastroepiploic vessels. It is of paramount importance to preserve these vessels because they form the most important blood supply to the stomach after mobilization. As one approaches the spleen, the division of the short gastric vessels can stay close to the stomach wall. The left crus is exposed when the short gastric vessels are divided and the fundus mobilized medially. The phrenoesophageal membrane is detached and the abdominal esophagus and cardia can be freed on the left side.
The gastrohepatic ligament is then detached from the liver and from the portal structures. In some cases, an aberrant left hepatic artery is seen arising from the left gastric artery. Although its ligation can usually be done with impunity, postoperative deranged liver function and even partial liver necrosis have been reported. When found to be sizable, the artery should be preserved. This can be accomplished by dissecting upward along the left gastric artery from the celiac axis and ligating vessels going into the lesser curvature of the stomach while preserving this vessel. Dissection from the right side toward the esophageal hiatus frees the right crus, and dissection anterior to the esophagus will meet the already dissected plane from the left. The anterior vagus nerve can be divided at this point. The esophagus is thus freed on both sides as well as anteriorly. A sling placed around the lower esophagus, such as a Penrose or latex drain, may help later dissection by providing retraction.
For a tumor of the distal esophagus located at the hiatal opening, especially for a transmural T3/T4 tumor, a cuff of diaphragmatic crura can be removed together with the tumor. Division of the hiatal musculature is begun on the left side. Bleeding from the lateral edge is controlled with suturing or electrocautery. Division is continued along the front and then to the right side until a cuff of muscle is freed. When a cuff of muscle from the esophageal hiatus is removed, both pleural cavities are likely to be entered, but this is of no serious consequence.
The stomach is then reflected upward and dissection is begun at the celiac trifurcation. Using fine electrocautery, dissection is performed along the anterior aspect of the common hepatic artery. This is usually easily performed. Lymphadenectomy can then proceed laterally toward the hepatoduodenal ligament. For a tumor of the intrathoracic esophagus, formal dissection of lymph nodes along the hepatoduodenal ligament (common bile duct and hepatic artery alongside) is not necessary. Medially toward the origin of the left gastric artery at the celiac axis, the left gastric or coronary vein requires ligation. The origin of the left gastric artery is identified separately as it comes off the celiac axis, and it is doubly ligated and cut between ligatures. Further dissection toward the left will clear the lymphatic tissues on the splenic artery. The areolar tissue thus superior to the common hepatic artery and splenic artery is cleared en bloc with the abdominal esophagus toward the hiatus. Continued dissection upward can be carried out through the esophageal hiatus along the front of the aorta. Areolar tissues are freed from the aorta and remain attached to the resected specimen. The cardia and abdominal esophagus are thus freed totally. With fine electrocautery and selective use of metal clips for small vessels, often the celiac trifurcation lymph node dissection can be a bloodless exercise.
On the lesser curvature, the right gastric vessels are divided at a convenient point (Fig. 3). This point, however, should be located distal to at least the third branch of the left gastric artery counting from its origin. Significant occurrence of lymph node metastases has been documented along branches going into the lesser curvature closer to the origin of the left gastric artery. The lesser omentum can be resected separately. The preparation of the lesser curvature makes subsequent transection of the
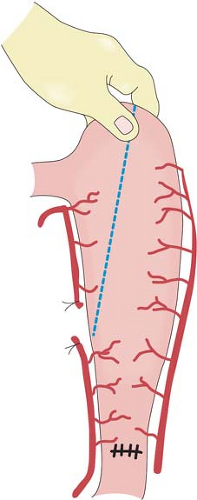
stomach easier during the thoracic phase of a Lewis-Tanner operation. When a three-phase esophagectomy is carried out, the stomach can be transected with linear staplers at this stage. The stomach is straightened and the highest point marked. The stomach is transected from this point toward the prepared site at the lesser curvature. A narrow gastric tube is made to be delivered up to the neck for esophagogastrostomy.
stomach easier during the thoracic phase of a Lewis-Tanner operation. When a three-phase esophagectomy is carried out, the stomach can be transected with linear staplers at this stage. The stomach is straightened and the highest point marked. The stomach is transected from this point toward the prepared site at the lesser curvature. A narrow gastric tube is made to be delivered up to the neck for esophagogastrostomy.
Many factors determine gastric emptying after esophagectomy, including the necessary vagotomy, the size of the gastric tube, and the route of placement of the conduit. Vagotomy in theory will inhibit gastric emptying, a smaller-size gastric tube drains better than a whole-stomach conduit, and a stomach brought up to the neck via the posterior mediastinum empties better compared to one in the right thoracic cavity because of the angulation at the hiatus. A Heineke-Mikulicz pyloroplasty is performed using a continuous running absorbable monofilament suture in two layers. The first layer coapts the mucosal and submucosal layers, and the second layer, using the same suture, incorporates only the seromuscular layers. This technique allows accurate apposition of individual layers with inversion but minimal infolding. A pyloromyotomy works equally well. Some surgeons opine that a pyloric drainage procedure will enhance enterogastric bile reflux. In the author’s experience, clinically significant symptomatic biliary gastritis is uncommon. A Kocher maneuver is not mandatory, provided the stomach is of sufficient length. However, this maneuver is easily performed, and does have the advantage of straightening the “axis” of the pyloroduodenal region when the stomach is brought up to the right thoracic cavity. After careful hemostasis, the abdomen is closed. Abdominal drains are not required.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree