Figure 43-1. Barium contrast esophagography showing the constriction caused by the cricopharyngeal muscle, aortic arch, and the left mainstem bronchus. Indentations of the esophagus made by external structures are important anatomic landmarks and are often the sites of perforation due to instrumentation.
2 The incidence of perforation has risen in the modern era due to the rise in endoscopic interventions. The causes of esophageal perforation are listed in Table 43-1. Generally speaking the incidence of iatrogenic perforation (59%) far outweighs the incidence of spontaneous perforations (15%).12 Other less common injuries include foreign-body ingestion (12%) and trauma (9%). Ingestion of foreign bodies or caustic materials most commonly causes perforations in the anatomic areas just proximal or at narrowing (cricopharyngeus, level of aortic arch, and the level of left mainstem bronchus). Also included in the area at risk is the lower esophageal sphincter zone. External penetrating trauma can occur at any level but is seen more commonly in the cervical esophagus as the mediastinal portion is posterior and protected by the thorax. The morbidity and mortality of penetrating esophageal trauma is usually related to associated injuries including major vascular and airway structures.16 Blunt traumatic perforation of the esophagus is exceedingly rare.
The most commonly referenced and discussed perforation is the spontaneous one. This results from a sudden increase in intraesophageal pressure and associated with hyperemesis, childbirth, seizure, or prolonged coughing. Boerhaave syndrome is of special historical note, wherein patients develop perforation of the distal esophagus following extensive ingestion of food or alcohol. What follows is violent emesis resulting in a distal esophageal perforation, frequently into the left chest. The syndrome was named after Herman Boerhaave, who provided a detailed account of this condition in 1723 through a postmortem correlation of the perforation found in the High Admiral of the Dutch Navy, Baron Van Wassenaer.17 Legend has it that the admiral attempted to relieve his postprandial discomfort by self-induced vomiting after having feasted on roast duck and beer.
ETIOLOGY
Table 43-1 Causes of Esophageal Perforation

Clinical Features of Esophageal Perforation
The presenting features depend on the location of perforation and the time interval from perforation to presentation. Underlying comorbidities may also have an influence on the presentation and the patient’s physiologic reserve. Patients with cervical perforation present with dysphagia, neck pain, dysphonia, and subcutaneous neck emphysema. Intrathoracic perforations present with signs and symptoms of mediastinitis as follows: chest pain, tachycardia, tachypnea, fever, and leukocytosis. Perforations of the intra-abdominal esophagus may present as an acute abdomen with signs and symptoms as follows: pain, tachypnea, tachycardia, fever, and leukocytosis. More significant perforations and longer intervals to presentation contribute to the features of systemic sepsis, shock, and rapid deterioration.
Diagnosis
The diagnosis of esophageal perforation needs to begin with the clinician and their index of suspicion. Any patient who presents with pain following an endoscopic intervention needs to be carefully evaluated for perforation. The initial workup involves careful history and physical examination, laboratory studies including leukocyte count, and chemistry panel for acidosis. Chest x-ray is a good screening test for other diagnoses in the differential. Contrast esophagography remains the gold standard for diagnosis as the study will diagnose and localize the site of the perforation. This study also gives important information about coexisting pathology of the esophagus and the degree of extravasation and its trajectory. Water-soluble contrast (e.g., Gastrograffin) is the initial agent utilized, followed by thin barium to enhance the sensitivity of the study. Water-soluble agents demonstrate 50% of cervical perforations and 80% of intrathoracic perforations.18 Thin barium will identify 60% of cervical and 90% of intrathoracic perforations.19 If there is a chance of aspiration or high concern of tracheoesophageal fistula, thin barium should be used from the outset because the hyperosmolar water-soluble contrast agents may cause rapid pulmonary edema and respiratory compromise.
Computerized tomography (CT) scan is very useful in the diagnostic evaluation of the patient with concern for esophageal perforation. Often a CT scan is the first test obtained following a chest x-ray as it affords the clinician the ability to narrow the differential diagnosis. CT may be useful for identifying the site of perforation and any associated mediastinal or pleural fluid collections. It is very common for a CT scan finding to prompt thoracic surgical consultation or transfer and there is much additional information about the patient’s overall status which is routinely utilized in the management plan. However, it should be emphasized that the barium swallow should be the procedure of choice unless there is a significant risk of aspiration.
Flexible esophagoscopy should be utilized liberally in the diagnostic evaluation of the esophagus and in the planning of treatment options, and in a significant number of cases it can also be used for intervention. The scope is utilized to directly visualize the area of perforation and identify any potential associated pathology. It has a sensitivity of over 95% and a specificity of 80% or even higher depending on the experience of the physician.20 We routinely perform flexible endoscopy as the initial step in the operating room when considering all options for patient management. Concern has been expressed by some that the instrumentation of the esophagus and insufflation of air can worsen the extent of injury and pathology and we would agree with that in inexperienced hands. With care and experience, however, the flexible scope can be used safely and more effectively delineates the problem to aid in surgical decision-making that follows. The vast majority of patients will require an endoscopic evaluation at some point and the timing of this will depend on clinical presentation and findings on radiologic imaging. For very small leaks present in stable and nontoxic patients, when the contrast study shows little or no extravasation, we may opt to hold off on an endoscopy in select cases.
Management
3 The treatment of esophageal perforation is managed according to the clinical presentation which includes the location of the perforation, the degree of soilage, and the clinical condition of the patient. Generally speaking, the sooner diagnosis and a treatment plan is carried out, the better the outcome. The goal of treatment is to stabilize the patient, stop ongoing soilage, control the infection, and reestablish esophageal continuity. Algorithm 43-1 provides general guidelines to the management of patients with esophageal perforations.
Nonoperative Treatment
For properly selected patients with esophageal perforation, nonoperative management may be appropriate. Generally, as thoracic surgeons, we err on the side of operative exploration intervention given the potential gravity of missing a clinically significant esophageal perforation with mediastinal soilage. Reports of nonoperative management date back to the 1960s in which successful management was accomplished in 18 highly selected patients with only one death.21 In the late 1970s, criteria were published for nonoperative management.22 Current guidelines for patients that can be managed nonoperatively include those with no transmural perforation or for whom transmural perforation represents a contained process. There must also be no associated esophageal pathology such as malignancy and no signs of systemic illness. The management of the nonoperative approach begins with the administration of broad-spectrum antibiotics (including antifungals), the suspension of oral intake, and vigilant clinical monitoring. The patient is followed clinically and reimaged as needed, generally within 48 to 72 hours with an esophagram and CT scan. If the patient improves during that time and testing is favorable, the diet may be advanced to clear liquids with the patient eventually being discharged on oral antibiotics and full liquids until reevaluation with imaging in clinic in 2 weeks. Any signs of sepsis such as fever, tachycardia, or leukocytosis suggest failure of this approach and further intervention should be expeditiously considered.

Algorithm 43-1. Evaluation and treatment of esophageal perforation.
Esophageal Stents
4 Endoscopic placement of endoluminal stents for perforations has been a concept which has been discussed for several decades.23 In the last 10 years, the technology of stents has improved such that deployment is easier and our indications have expanded to include placement for perforations. Starting with small limited series,24–30 their use has expanded dramatically to the point that they have become one of the main tools in the management of select, contained perforations. Highly selected patients with perforations are candidates for stenting in the context of a thoracic surgical practice according to the principles outlined in this section. Our own investigations and use in our practice has found that esophageal stent placement for perforations facilitates source control, may minimize stricture formation, and frequently allows for early oral intake. However, success depends on a uniform approach that focuses on appropriate patient selection, proper stent placement technique, thorough drainage procedures which cannot be underemphasized, and meticulous postoperative care. Our recommended uniform approach is presented here and this approach has yielded excellent results.31–33
DIAGNOSTIC EVALUATION
A computerized tomography (CT) scan, with or without oral and intravenous (IV) contrast, is a good initial diagnostic test. The role of a CT scan is to help determine whether additional source-control measures are needed (e.g., tube thoracostomy, decortication). We frequently perform the contrast esophagram second as the barium may interfere with the CT scan and the only reason we omit this step is in the case of aspiration risk. The barium esophagram is regarded as a real-time image, whereas the contrast delivery of the CT scan does not provide the same functional and anatomic details of the site and tracking of the leak, thus we prefer both when possible.
PATIENT SELECTION
For perforations proximal to the cricopharyngeus, stents have little or no role. For perforations 2 cm or more distal to the cricopharyngeus, a stent may work if the proximal extent seats just below the cricopharyngeus muscle. If the stent seats at or above the cricopharyngeus, patients experience intolerable foreign-body sensations during deglutition and reflux and coughing are generally severe. Also of note, stents placed in the proximal esophagus may cause pressure and compression of the posterior membranous trachea leading to dyspnea and, in severe cases, critical airway compromise. If a stent is considered in the proximal esophagus, the physician should strongly consider performing a simultaneous bronchoscopy to assess airway patency. Perforations below this area may be good candidates for stenting when there is limited perforation, and there is good purchase of esophagus above and below the site of the perforation. This might include perforations associated with malignancy and in benign diseases such as iatrogenic perforation, Boerhaave syndrome, and select cases of achalasia. Patients with perforations from near-obstructing cancers can be ideal candidates for stents as the stent will purchase well against the tumor, leading to sealing of the perforation and relieving any coexisting blockage above or below the perforations. In addition, operating on perforated cancers can be a significant clinical problem as this may occur at the initial diagnosis or the endoscopic ultrasound (EUS), or may occur during chemo and radiation, thus making stenting our procedure of choice in the setting of cancer.
Initial broad-spectrum antimicrobial therapy is directed to cover common gram-negative bacteria, anaerobic bacteria, and fungi. We generally use a beta-lactam agent in combination with fluconazole, which is usually sufficient. We adjust therapy to culture findings. Duration of therapy is patient dependent, but it is typically continued for 10 to 14 days after resolution.
In addition to antimicrobial therapy, we tailor further source control in accordance with clinical, CT scan, endoscopic, and intraoperative findings. We emphasize aggressive source control, and operative drainage of any extraluminal material. Stent placement without drainage in the setting of mediastinal soilage is a risky approach and if it is done, one may question whether the perforation was small enough to need intervention and, if larger, we would typically perform a VATS or thoracotomy to debride any soilage. If patients deteriorate clinically following any treatment, consideration of the presence of undrained sepsis should always be considered. At times, a patient might require several interventions to control the local and regional sepsis.
Source control measures vary depending on clinical and anatomical findings:
Mediastinal air (no fluid): antibiotics only without additional source control measures.
 Mediastinal fluid collection or abscess: operative drainage; the approach varies by anatomical location:
Mediastinal fluid collection or abscess: operative drainage; the approach varies by anatomical location:
 Neck and superior mediastinum: lower neck incision with blunt mediastinal dissection, irrigation, open packing, and, if deemed appropriate, drain placement.
Neck and superior mediastinum: lower neck incision with blunt mediastinal dissection, irrigation, open packing, and, if deemed appropriate, drain placement.
 Posterior mediastinum at any level: right thoracoscopy or thoracotomy with wide pleural incision, drainage, irrigation, and large-bore chest tube(s) placement.
Posterior mediastinum at any level: right thoracoscopy or thoracotomy with wide pleural incision, drainage, irrigation, and large-bore chest tube(s) placement.
 Lower posterior mediastinum only: left thoracoscopic or open drainage.
Lower posterior mediastinum only: left thoracoscopic or open drainage.
 Free-flowing pleural effusion: large-bore tube thoracostomy.
Free-flowing pleural effusion: large-bore tube thoracostomy.
 Empyema: thoracoscopic or open decortication.
Empyema: thoracoscopic or open decortication.
STENT SELECTION
Numerous stents are available for use. We prefer the Wallflex fully covered stent and find that the longer stents (15 cm) work better to cover defects and minimize migration.
STENT PLACEMENT TECHNIQUE
We perform our procedures in the operating room and with the patients under general endotracheal anesthesia. The key components of our stent placement technique are the following:
1. Position: supine position with the head of the bed elevated 30 degrees.
2. Intraoperative fluoroscopy:
 Radiopaque skin markers: large-bore IV needles taped to the skin. Lock the fluoroscopy arm into position once the desired image has been obtained and before skin marker placement; moving the fluoroscopy arm after marker placement may lead to inaccurate marking.
Radiopaque skin markers: large-bore IV needles taped to the skin. Lock the fluoroscopy arm into position once the desired image has been obtained and before skin marker placement; moving the fluoroscopy arm after marker placement may lead to inaccurate marking.
 At times, we will use intraoperative esophagram: if the leak is small, we will inject contrast through the esophagoscope to clearly identify it; we always confirm leak sealing with contrast injection after stent placement. We use isomolar and water-soluble contrast dye (iodixanol [Visipaque]; GE Healthcare Inc, Princeton, NJ) to minimize respiratory complications in case of aspiration.
At times, we will use intraoperative esophagram: if the leak is small, we will inject contrast through the esophagoscope to clearly identify it; we always confirm leak sealing with contrast injection after stent placement. We use isomolar and water-soluble contrast dye (iodixanol [Visipaque]; GE Healthcare Inc, Princeton, NJ) to minimize respiratory complications in case of aspiration.
3. Wire of choice: superstiff, angled or straight tip, 0.035-in (0.8-mm) diameter, 260-cm length.
4. Stent position:
 Minimum coverage: at least 3 to 4 cm above and below the leak or perforation.
Minimum coverage: at least 3 to 4 cm above and below the leak or perforation.
 Minimum distance from cricopharyngeus muscle (upper esophageal sphincter): 1 to 2 cm.
Minimum distance from cricopharyngeus muscle (upper esophageal sphincter): 1 to 2 cm.
 Distal end position: we avoid the distal end from crossing the GE junction.
Distal end position: we avoid the distal end from crossing the GE junction.
5. Stent foreshortening or “jumping” on deployment: this varies by stent.
6. Intraoperative evaluation of stent position and proper sealing: every stent is placed and evaluated with fluoroscopic guidance and endoscopy.
7. Tools and techniques for stent repositioning (if stent is too distal or too proximal): the “rattooth” forceps is the best tool to pull the stent proximally if needed after deployment. If we need to advance the stent in a patient with a gastric conduit, then we will advance the gastroscope into the distal stomach, retroflex, lock the “rat-tooth” grasper on the distal end of the stent, and push the gastroscope forward.
8. Indications for immediate stent removal: we never leave a stent in place if it is not in perfect position, angulated too much, or obstructed.
POSTSTENT MANAGEMENT
1. Nutrition: enteral feeding access should be ensured as soon as possible, preferably at the time of endoscopic and operative interventions. We place an operative jejunostomy tube or percutaneous endoscopic jejunostomy tube in the occasional postesophagectomy patient who does not already have enteral feeding access. In patients with perforations, we place a percutaneous endoscopic gastrostomy (PEG) tube at the time of endoscopic or operative intervention but before stent placement. In our experience, placing a PEG tube has no effect on the perforation; placing a PEG tube after stent placement will likely displace the stent.
2. Aspiration and reflux precautions: these precautions are imperative in all postesophagectomy patients and in patients with stents that cross the gastroesophageal junction.
3. Postprocedure follow-up:
a. Chest radiograph: we obtain chest radiography immediately after the procedure and daily thereafter to monitor for migration.
b. Sepsis resolved: we wait until the patient is able to swallow appropriately; then we perform an esophagram.
c. Ongoing sepsis: we thoroughly reassess the patient; ongoing sepsis is generally due to poor source control and/or nonsealing of the leak or perforation. Reintervention is then tailored to the situation but might require aggressive surgical intervention and stent removal and/or replacement.
d. Stent migration: mandates replacement unless leak has resolved. This is typically not an emergency but should be addressed in a timely fashion.
4. Resumption of oral intake: this depends on the clinical scenario. A speech pathologist evaluates every patient before an esophagram. Only patients who are safe to swallow undergo an esophagram. Oral intake is then resumed.
5. Eventual stent removal or exchange: We generally repeat endoscopy at 3-week intervals and remove the stent. If a small leak persists, we restent so that the distal end of the stent is slightly proximal or distal to that of the previous stent to allow the area of inflamed gastric mucosa to heal.
6. Pain: pain appears to be less common in patients with leaks and perforations than in patients with malignant strictures. Persistent pain should prompt reevaluation, because it might represent ongoing mediastinal or pleural contamination.
Operative Treatment
The operative management of esophageal perforation is dictated by the location of the injury, extent of injury, and underlying pathology. The operative approaches include the following options: drainage alone, primary reinforced repair, esophagectomy with immediate/delayed reconstruction, and esophageal exclusion.
Perforations of the upper third of the esophagus to the level of the carina and treated by cervical drainage that is approached via a left neck incision as depicted in Figure 43-2. Traditionally it is not necessary to find or close the perforation as this will seal during the process of healing. In order for this to be successful, however, wide and complete drainage must be accomplished. The posterior prevertebral fascia is opened completely to accomplish this. Preoperative imaging via CT scan will aid in ensuring that the important areas of contamination are addressed surgically. In the operating room we liberally use on-table flexible endoscopy to evaluate the defect and the location of the hole. If the hole is identified and it is small, we advocate for closure of the hole. The neck incision is loosely closed with staples over a drain. Postoperatively, broad-spectrum antibiotics are continued. One may consider a gastrostomy or jejunostomy tube for nutritional access during the time the patient is NPO. Typically at 5 to 7 days postoperatively a contrast esophagram is obtained, the patient’s diet is advanced, and the drain is removed. If there is continued leak, the patient is left NPO or on clear liquids and restudied in 1 week. There should be no distal obstruction on the contrast study and if there is a stricture that prevents normal contrast flow, we advocate for dilation to aid in eventual closure of the esophageal fistula. As long as there is no underlying pathology and there is distal flow through the esophagus in the presence of adequate drainage, the esophageal perforations will heal spontaneously.
Perforations of the middle third of the esophagus are best approached through the middle third of the esophagus via a right 5th interspace posterolateral thoracotomy. After adequate IV access and arterial line placement, a single-lumen tube is placed and bronchoscopy/esophagoscopy is performed. The single-lumen endotracheal tube is changed to a double-lumen endotracheal tube and the patient is positioned in the left lateral decubitus position. We routinely start thoracoscopically, but typically these patients are very ill and thoracotomy is indicated early. At the time of entry into the chest, an intercostal m. flap is routinely taken. We use papaverine solution to enhance blood flow and great care is taken not to injure the flap when the retractors are placed. A Doppler is routinely used to confirm blood flow in the flap. All pleural collections are drained, the lung is decorticated, and the site of perforation identified after opening the posterior mediastinal pleura. Necrotic tissue at the site of the perforation is debrided and the esophagus is mobilized. A vertical myotomy is performed to expose the mucosa and the full extent of the tear (Fig. 43-3). The mucosa is repaired with interrupted or running absorbable suture. The muscular layer is reapproximated with interrupted fine silk suture. On-table endoscopy is performed to ensure the leak is repaired. The intercostal m. is then secured as an onlay patch. Large chest tubes are placed and a 10-French Jackson–Pratt drain is left in the area of the repair and placed to bulb suction. Typically nutritional access is not addressed at the time of the index operation as the patients are quite ill, and this can be accomplished at a later date. On POD 7, a contrast study is obtained and if there is no leak, the diet is advanced. Persistent leaks are treated with JP drainage, provided CT imaging demonstrates that there are no undrained collections. Esophageal dilation may be required to ensure proper forward flow. Reoperations for adequate drainage may be required. Esophageal stenting may be used to exclude the leak provided that there is adequate drainage and the principals described in the stenting portion of this chapter are followed. If the repair breaks down or there are signs of clinical sepsis, esophageal exclusion may be required with cervical esophagostomy, esophageal resection, and gastrostomy tube. When the patient recovers and walks into clinic begging for reconstruction (this usually occurs at 6 to 12 months postexclusion), they may be considered for reconstruction most commonly approached as a substernal gastric pull-up.

Figure 43-2. Approach for drainage of a cervical esophageal perforation. A: Skin incision parallel to the anterior border of the left sternocleidomastoid muscle, extending from the level of the cricoid cartilage to the sternal notch. B: With the sternocleidomastoid muscle and carotid sheath retracted laterally and the trachea and thyroid gland medially, blunt dissection along the prevertebral fascia in the superior mediastinum is carried out. Injury to the recurrent laryngeal nerve in the tracheoesophageal groove must be avoided. C: Schematic drawing of the prevertebral space drained by this cervical approach. D: Two 1-in rubber drains placed into the superior mediastinum are brought out through the neck wound to allow establishment of an esophagocutaneous fistula, which usually heals spontaneously.
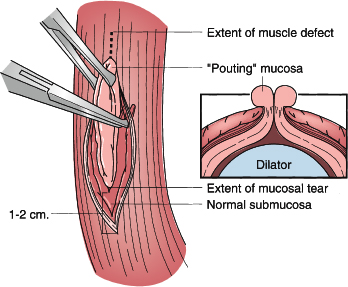
Figure 43-3. Primary repair of esophageal perforation. The edematous mucosa pouting through the muscular defect (inset) is grasped with Allis clamps and elevated. A 1-cm vertical esophagomyotomy is made at either end of the muscular defect to expose the entire limits of the tear. This is facilitated by using a right-angle clamp to direct muscularis away from underlying submucosa around the entire circumference of the tear. The result of this mobilization is exposure of a circumferential rim of normal submucosa that can then be closed.
Perforations of the distal third of the esophagus are approached via a left 7th intercostal space thoracotomy. Similar to the approach for middle-third perforations, after adequate IV access and arterial line placement, a single-lumen tube is placed and bronchoscopy/esophagoscopy is performed. The single-lumen endotracheal tube is changed to a double-lumen endotracheal tube and the patient is positioned in the left lateral decubitus position. Thoracoscopy may be an option, but typically these patients are very ill and thoracotomy is indicated. At the time of entry into the chest, an intercostal m. flap is routinely taken and care is taken not to injure it as retractors are placed. The chest is drained, lung decorticated, the perforation identified, and the repair performed as described above.
The operative treatment in the presence of underlying pathology deserves special attention and can be quite challenging. If there is distal obstruction, this must be dealt with. Strictures can be dilated or stented and often support the healing of a proximal perforation. Patients with achalasia who are perforated during pneumatic dilation are especially challenging. Many are candidates for nonoperative intervention as they have small perforations that are asymptomatic and the patients are clinically nontoxic. This is acceptable provided the distal obstruction has been relieved. For those who fail nonoperative management or are not candidates, operative management should be tailored to the individual and their pathology. In patients with preserved motility, the perforation should be repaired primarily and a vertical myotomy should be performed 180 degrees opposite the injury. These patients should have a nonobstructive fundoplication performed. For stable patients with advanced achalasia, esophagectomy should be considered. For unstable patients, esophageal exclusion should be performed.
Unstable patients with advanced pathology such as cancer should undergo esophageal exclusion. This includes stapled esophageal diversion with resection of the diseased segment of the esophagus. A linear stapler is deployed on the esophagus proximally and at the gastroesophageal junction. The esophagus is resected. An end cervical esophagostomy is created saving as much esophageal length as possible. Pleural drainage is accomplished. Subsequently the patient undergoes placement of gastrostomy (+/– jejunostomy tube) with repair of the diaphragmatic defect from the abdominal side via laparoscopy or laparotomy. The components of operation may be staged based on the severity of patient illness. If the patient survives and is a candidate for reestablishment of continuity, this can be investigated at a later date when they recover. In the current healthcare climate, the presentation of an advanced perforated malignancy, stenting and drainage may have a role. Additionally, consideration should be given to end-of-life discussions depending on the extent of the cancer.
Caustic Injury
Caustic burns of the esophagus result from ingestion of caustic substances. In children, ingestion is typically accidental, but in adults, ingestion is often intentional as part of a suicide attempt. Although prevention programs, consumer product safety commissions, lobbying by the medical profession, and heightened consumer awareness have significantly diminished the devastating effects of caustic ingestions, caustic injury of the esophagus remains a significant medical and social problem. The two most common ingestion scenarios are accidental ingestions in children younger than 5 years old and suicide attempts in individuals 15 to 40 years old. The most commonly ingested agent is an alkaline household liquid cleaner. Although mortality from caustic ingestion is low, the morbidity associated with caustic ingestions is significant. Acute morbidities include perforation and necrosis, and late morbidities, which are more prevalent, include stricture formation and cancer.
The extent of injury to the esophagus caused by ingested caustic material is dependent on the volume and nature of the ingestion. Injuries resulting from ingestion of liquids are much more severe than those caused by ingestion of solids. The patient usually spits particulate matter out and, thus, it rarely moves beyond the oropharynx. Liquid caustic agents contribute to pathology down the length of the esophagus. Strong acid and alkali ingestions comprise the majority of caustic ingestions. These substances are sold in various liquid and solid/particulate forms. The most commonly ingested strong acids include industrial and swimming pool cleaning solutions, battery fluids, and antirust compounds. Commonly ingested strong alkaline-containing substances include household cleaning products and personal hygiene products. The most commonly ingested weak alkali is ammonia hydroxide, which is found in most bleach solutions. Weak alkalis cause much less injury and require far less therapy.
The burns from acid ingestion cause coagulative necrosis and a superficial eschar that protects the deeper layers of the esophagus from damage. Fortunately, acids tend to taste bitter and cause immediate pain, thus the patient will usually spit the substance out well before it reaches the esophagus and stomach. When acids are ingested in liquid form, they move quickly, typically spare the oropharynx, and produce skip injuries to the esophagus. Acids in liquid form cause gastric and duodenal injury more frequently than esophageal injury.
Alkaline burns are characterized by liquefactive necrosis, leading to far greater penetration at the level of the oropharynx and esophagus. Solid alkali has a propensity to adhere to the oropharynx, whereas liquid alkali is rapidly swallowed. Thus, liquid alkali results in more distal esophageal and gastric injury. Frequently there is penetration of all layers of the esophagus causing substantial secondary edema. Commensurate with this, the potential for perforation and circumferential scar formation is greatest with an alkaline ingestion. In contrast to acid ingestion, alkali substances are typically odorless and tasteless, which enhances the potential for deeper injury.
Management
The initial clinical presentation comprises oropharyngeal pain; irritability; and, in severe cases, excessive salivation, dysphagia, and airway compromise. Ingestion of particulate caustic substances results in signs of contact readily visible in the oropharynx. Hoarseness and stridor are signs of serious airway injury that mandate intubation. Retrosternal pain, neck crepitus, and sudden onset of epigastric pain imply full-thickness esophageal damage. Many patients present without symptoms and a significant proportion have injuries that will require intervention. Thus, a 24-hour period of observation is reasonable, even for asymptomatic patients, especially if there is any question about the circumstances of the ingestion or extent of injury.
As with any acute situation, management should begin with the standard assessment of the ABCs (airway, breathing, and circulation) (Algorithm 43-2). Most caustic ingestions involve the oropharynx, lips, and gums. As with most foreign-body ingestions, the most common sites for pathology within the esophagus are its three areas of narrowing: the cricopharyngeus, the level of the aortic arch, and the gastroesophageal junction. A stable patient may undergo imaging via posteroanterior and lateral upright chest and abdominal radiographs to evaluate for pleural effusion or free air. CT of the chest and abdomen can provide important anatomical definition of injury. Patients with no visible symptoms or only lip swelling or redness can be observed in the hospital or a short-stay unit for 24 hours to ensure they can tolerate oral feedings. This is typically for pediatric patients. Patients with oral lesions should undergo esophagoscopy and bronchoscopy. The bedside insertion of a nasogastric or orogastric tube is discouraged when the extent and depth of injury have not been fully evaluated.
After radiographic imaging, rigid or flexible esophagoscopy to characterize the location and extent of injury should be the next course of investigation. This instrumentation should be performed within the first 24 hours as the risk of procedural complication increases with time. The indications for esophagoscopy include stridor, vomiting, drooling, and intentional ingestion. Caution should be used when moving beyond the first sign of pathology below the cricopharyngeus. If there are respiratory symptoms, laryngoscopy and bronchoscopy should be performed. When injury is identified, the standard grading of the esophageal burn should be reported (Table 43-2).
Patients with grade I injuries have superficial burns causing edema and hyperemia. These patients can be observed, a normal diet can be advanced, and the patient ultimately can be followed in an outpatient clinic. Superficial grade I injuries cause mucosal sloughing and should heal without a stricture. Grade II injuries penetrate into the muscular layer of the esophagus and can result in either patchy (IIA) or circumferential (IIB) injury. Ulcerations (superficial and deep) and pseudomembranes are found during endoscopy. Once circumferential injury is identified, the risk of stricture formation increases dramatically. Grade III injuries are full-thickness injuries, and mediastinitis or peritonitis occurs within 48 hours. Grade III findings are characterized by gray mucosal slough, thrombosed submucosal vessels, and black eschar. All grade III burns and >75% of circumferential grade IIB burns cause esophageal stricture and, as expected, are associated with a higher incidence of infection.

Algorithm 43-2. Proposed algorithm for evaluation and management of acute caustic ingestion.
Acute management of caustic ingestion should be dictated by the endoscopic grading of the esophagus and the clinical status of the patient (Algorithm 43-2). Patients should be evaluated for using endoscopy to grade the injury. If there is no evidence of perforation, patients with mild exposure (e.g., bleach or detergent ingestion) can be observed for 24 to 72 hours (depending on the clinical presentation). Generally, patients should be kept nil per os (NPO) until pain free. Enteral feedings can likely be introduced within 48 hours for all patients with grade I or IIA burns.
CLASSIFICATION
Table 43-2 Endoscopic Grading of Acute Caustic Injury to the Esophagus

Patients with grade IIB or grade III esophageal burns without evidence of perforation should be placed in a monitored setting, kept NPO, and given IV fluids and IV antibiotics (penicillin derivative or clindamycin). Patients should also be maintained on proton pump inhibitors to reduce acid exposure of the esophagus as this may decrease the stricture incidence. Steroid administration is controversial, does not prevent stricture formation, and may mask findings in the serial abdominal examination. If corticosteroids (2 to 2.5 mg/kg/d) are used, they should be instituted within the first 24 hours of ingestion and for 3 weeks thereafter. Early introduction of parenteral nutrition is essential for all patients with esophageal perforation and for patients with poor gastric motility. Open gastrostomy is helpful for patients with grade IIB or III burns. Depending on the extent of injury, the patient may require mechanical ventilation. Esophagram (or CT scan with oral contrast) may be used as an adjunct evaluation. If free contrast extravasation into the mediastinum is visualized, drainage of the mediastinum into the pleural space is indicated. Further, esophageal resection may be required with findings of necrosis. On occasion, a contained mediastinal leak, which returns contrast material back into the esophagus, may be observed without the need for immediate drainage. Pneumoperitoneum is an absolute indication for laparotomy and resection of all dead tissues. Laparoscopic evaluation may be useful to evaluate the abdomen if there is isolated mediastinal pathology.
Strictures of varying degrees occur in almost all patients and, thus, careful follow-up is recommended after a caustic ingestion. The esophagus and stomach should be evaluated via barium swallow 3 weeks, 3 months, and 6 months after the ingestion to rule out stricture formation or gastric outlet obstruction. Esophagogastroduodenoscopy (EGD) is typically performed 3 weeks after the ingestion for full evaluation. Early endoscopy is warranted during the phase of cicatrix formation, and dilation is repeated at varying intervals. The interval for intervention is patient dependent and clinically dictated by the amount of dysphagia or interval to recurrence of dysphagia, the initial severity of burn, and findings on radiographic studies.
Operative Considerations
Delay in the diagnosis of esophageal perforation from caustic ingestion is uniformly fatal. Isolated mediastinal air can be managed with direct inspection of the thorax, open débridement of necrotic tissue, and drainage of the mediastinum. Caustic esophageal perforations are best managed with urgent esophagectomy. Removal of the entire esophagus is possible through a transhiatal approach followed by a cervical esophagostomy, gastrostomy, and feeding jejunostomy. Patients with extensive full-thickness esophageal and gastric necrosis should undergo total esophagogastrectomy with cervical esophagotomy and feeding jejunostomy. Of course, any operative intervention should include assessment of other organs involved and complete drainage/resection of necrotic tissue.
In most patients, management of esophageal strictures that develop as a result of caustic burns is the primary operative consideration. Circumferential grade IIB burns can be managed with an open gastrostomy and insertion of a string through the gastrostomy that exits transnasally. Four weeks after the burn, serial dilations using progressive antegrade bougienage from the oropharynx or retrograde stringing bougienage through the gastrostomy is effective. Retrograde string bougienage or antegrade dilations should be performed at 2- to 3-week intervals for consecutive dilations. Optimal caliber bougienage is 48 to 50 for adults and 34 to 36 for children. Esophageal stenting is a newer approach for second-degree esophageal burns, which may be more palatable than string bougienage. A removable, fully covered stent is placed endoscopically and left in place for 3 weeks. It can then be removed. Recent reports have suggested that this is an appropriate intervention to prevent stricture formation in some patients. Injection of the stricture with triamcinolone (30 mg/mL) also may be effective, especially in those strictures that are focal.
Esophageal replacement is usually considered if a stricture fails to resolve after 1 year of dilations, stricture injections, and antacid therapy. Malignant degeneration is possible at the area of the stricture, and esophageal cancer occurs at a 1,000-fold greater frequency in individuals with a caustic injury to the esophagus than in the general population. Any change in symptoms warrants radiographic and endoscopic evaluation. Options for esophageal replacement include reverse gastric tube, gastric pull-up, and colon or jejunal interposition. Most centers today use the gastric pull-up or colon interposition (retrosternal). Short-term complications of replacement are those typical of esophagectomy; anastomotic leak and poor conduit vascularity are the most feared complications. Long-term complications depend on the type of replacement. Stomach conduits have the problem of continued gastric acid secretion and the possibility of reflux and dysplasia. The colon (and the jejunum) can become redundant and require revision. Overall, there is no optimal substitute for one’s own esophagus and efforts should be made to preserve it at all costs.
Foreign Body
Foreign-body ingestion and food bolus impaction are common occurrences that physicians can encounter when dealing with the esophagus. A majority of foreign bodies have been known to pass through the gastrointestinal tract spontaneously. However, roughly 10% to 20% of cases may require nonoperative intervention, with <1% requiring surgery.34–36 A large percentage of objects that do not pass spontaneously remain in the cervical esophagus.
Foreign-body ingestion is most commonly seen in children between the ages of 6 months and 3 years, with over 100,000 new cases occurring in the United States annually.37 Pediatric foreign-body ingestion is typically accidental and most frequently involve coins, followed by toy parts, batteries, bones, and food.37 Cases of foreign-body ingestion in adults are seen more commonly among the psychiatric patients, patients with impaired cognition such as the elderly, and incarcerated individuals seeking secondary gain.34–36
Endoscopy is the choice of management when nonoperative intervention is a viable option. Timing of endoscopy is indicated by the risk of esophageal wall damage or aspiration, as well as the clinical status of the patient. Urgent intervention using endoscopy may be required in patients with severe distress, unable to swallow their own secretions, or with foreign bodies that are batteries or have sharp edges. Patients who do not fit these criteria and have no evidence of severe obstruction can be handled less urgently as most foreign objects will pass spontaneously.
Retrieval is the goal when dealing with foreign bodies that are not subjected to spontaneous passing. General anesthesia is recommended during the entirety of this procedure. Flexible endoscopy is the preferred method for most foreign bodies. A rigid endoscope is useful in the retrieval of foreign bodies lodged in the proximal esophagus at the level of the cricopharyngeus muscle. Rigid esophagoscopy is a rarer procedure and one should be familiar with the technique. Often senior surgical help should be sought to accomplish the procedure safely and effectively because this technique is rarely used in current training. Using either approach, the object should be reoriented with a grasper and removed safely. When using the flexible esophagoscope, we have found the overtube to be a very useful tool for removal of foreign bodies such that the esophagus is not injured proximally upon extrication of the object. Esophagotomy may be required when the lodged foreign bodies are large, have sharp edges, are embedded in the mucosa, or exceed the diameter of the rigid endoscope in all orientations. Following removal of the foreign body, completion esophagoscopy and contrast esophagoscopy should be performed to rule out injury. If there is a perforation, it should be managed according to the algorithm in Algorithm 43-1.
BENIGN NEOPLASMS
Table 43-3 represents a list of all benign neoplasms of the esophagus. All of these lesions are rare, with leiomyoma making up roughly 60% to 70% of all cases.38,39 Esophageal polyps are also seen frequently in large referral centers and will be discussed. Other listed benign neoplasms of the esophagus will not be discussed in this chapter, but the possibility of an occurrence should be noted, especially during the formulation of a differential diagnosis.
Leiomyoma
As mentioned above, leiomyomas are by far the most common benign neoplasms of the esophagus, accounting for roughly 60% to 70% of these cases.38,39 The incidence of leiomyoma of the esophagus reported in autopsy range from 0.005% to 5.1%.40 Histologically, approximately 80% of leiomyoma originates from the muscularis propria and are located intramurally. Upon visual inspection of the esophagus, they are typically located in the middle and lower esophagus. They have been reported to present as single lesions. Because leiomyomas are slow-growing tumors, the size of these lesions often do not change for many years. Over 50% of these patients often are asymptomatic due to the nature of the growth.39
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree



