Fig. 7.1
Two different modes for gene transfer in articular cartilage regeneration: the direct in vivo method and the indirect ex vivo method
7.3.2 Vector Types for Cartilage Gene Transfer
7.3.2.1 Viral Vectors
In recent years, viral vectors and non-viral vectors have been commonly used for cartilage gene transfer. The adenovirus vector is most commonly used for its high transfection efficiency [44]. This vector can facilitate the stable and efficient transfection of a variety of cells but has a high immunogenicity in in vivo repair. The adenovirus vector also exhibits high toxicity at high doses. The toxicity of the adenovirus-associated virus (AAV) is milder than that of the adenovirus and has not been found to cause disease in humans thus far [45]. There is no viral protein expression in infected cells, but the transfection efficiency is not ideal. Adachi et al. used a retrovirus to transfect chondrocytes and stem cells in vitro, which were then embedded in a type I collagen gel and transplanted into the cartilage defect [46]. After 4 weeks, good histological results were obtained for the tissue repair in both groups. Nixon et al. used an adenovirus to transfect an IGF-1 gene that promotes secretion of a cartilage matrix into chondrocytes, bone marrow stem cells, and synovial cells, which were then filled into an articular cartilage defect [47]. The results showed that the aforementioned cells successfully prolonged the IGF-1 gene expression time and promoted the secretion of the cartilage matrix; however, a high initial virus titer was required, which had adverse long-term effects on the cartilage tissue. Note that in the aforementioned studies, all of the vectors used for gene transfection were viral vectors, which were chosen for their high transfection efficiency and ease of manipulation; however, the high immunogenicity of the viral vectors is as yet unresolved, which has limited their application to the clinical cartilage gene transfer system [48].
7.3.2.2 Non-viral Vectors
A technique has been developed that successfully uses non-viral vectors to elucidate gene structure, function, and expression. The preparation of a safe and efficient non-viral vector can profoundly impact the future development of gene therapy and biotechnology. Several polymers have been used for DNA transfer since the early 1970s; liposome is the most remarkable example of these polymers [49]. In 1987, Felgner et al. synthesized a cationic liposome, lipofectin [50]. The lipofectin/DNA complex is easy to manipulate and was the first vector used for in vivo chemical gene transfer. The peptide vector, in the form of the polylysine peptide vector, also has a strong affinity to DNA [50]. Consequently, the polylysine peptide vector/DNA complex can enhance the rate of cellular uptake. Polyethyleneimine (PEI) can inhibit lysosomes; in the acidic environment of endocytosis, PEI is protonated with an increased positive charge, which provides greater protection for DNA and facilitates plasmid escape from the lysosomes [51]. Consequently, PEI is widely used as a DNA transfer vector. Non-viral vectors are easy to manipulate for transfection and have a low immunogenicity and a high safety level; however, these vectors have a low transfection efficiency, and there target gene is only transiently expressed (typically for less than a week) [52]. Therefore, non-viral vectors are generally used only for in vitro mesenchymal stem cell differentiation and are difficult to use in vivo. Currently, an improved approach is being used in which the scaffold itself serves as a plasmid DNA transfer vector. The application of GAMs was developed to improve the poor efficiency of non-viral vector via maintaining the high local gene concentration and sustainingly delivering therapeutic DNA to surrounding cells. The first report to be described for bone used scaffold comprising a collagen sponge impregnated with plasmid DNA encoding for the BMP-4 gene with or without another plasmid encoding a portion of the parathyroid hormone gene, PTH1-34. It was designed to deliver DNA to infiltrating reparative cells when implanted into an osseous defect. By expressing the transgene, the infiltrating cells generate an autocrine and paracrine osteogenic environment. Satisfactory therapeutic effect was observed in experimental defect models in rats and dogs [53, 54].
Another example is that poly-cationic polymers (chitosan and gelatin) can bind to the negatively charged plasmid DNA; this matrix is itself biodegradable, and its degradation products can form complexes and coat plasmid components to form a DNA/polymer complex. Meanwhile, the plasmid DNA attached to the matrix surface is also continuously released as the matrix degrades, thereby improving the transfection efficiency and ensuring the continuous expression of the target growth factors. Guo et al. prepared a gene-activated chitosan/gelatin scaffold embedded with a TGF-β1 plasmid to effectively promote the proliferation of rabbit articular chondrocytes in vitro while maintaining the cartilage phenotype [55]. This gene-activated scaffold has the potential to become a new cartilage repair scaffold. Building Guo et al. work, Diao et al. transplanted bone marrow mesenchymal stem cells into the aforementioned scaffold to promote the differentiation of directional mesenchymal stem cells and the synthesis of a cartilage extracellular matrix; the active cartilage repair matrix, which had been constructed in vitro, was then transplanted into rabbit articular cartilage defects to repair the cartilage defects in vivo; favorable therapeutic results were obtained [56].
Chen et al. used two plasmids, TGF-β1 and BMP-2, together for the bidirectional differentiation of bone marrow mesenchymal stem cells into chondrocytes and osteoblasts; an osteochondral transplantation complex was constructed on the same scaffold. The authors simulated a bone and cartilage-like tissue for both bone repair and cartilage function in vitro, which was subsequently used to repair a full-layer osteochondral defect; the tissue in the surface hyaline cartilage and the subchondral bone were simultaneously repaired successfully [57]. The schematic diagrams of constructing the bilayered GAM and the therapeutic effect are shown in Fig. 7.2.
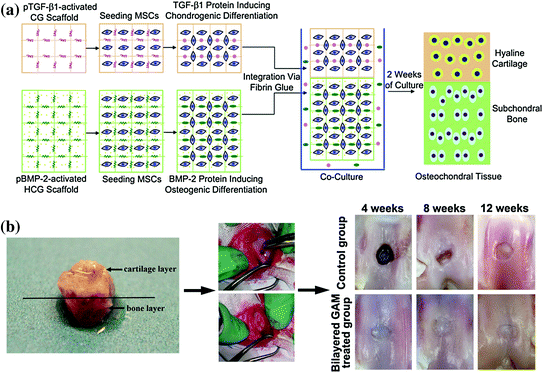

Fig. 7.2
a Diagrammatic representation of the procedures for the construction of the bilayered gene-activated composite osteochondral graft using mesenchymal stem cells loaded into TGF-β1-activated CG scaffold layer and BMP-2-activated HCG scaffold layer. pTGF-β1 plasmid TGF-β1; pBMP-2, plasmid BMP-2; MSC mesenchymal stem cell; CG chitosan–gelatin; HCG hydroxyapatite/chitosan–gelatin. b Osteochondral defects were created in the middle of each patellar groove of adult rabbits with a cylindrical drill. The bilayered gene-activated composite osteochondral graft was filled in the contralateral defect. Macrophotographs of the osteochondral repair in vivo were taken at 4, 8, and 12 weeks after the operation. Reproduced from [57]
7.3.3 Cell Types in the GAM
The cells used with a GAM for cartilage repair must have a stable source and a specified tissue repair potential. Currently used cells include adult chondrocytes, bone marrow mesenchymal stem cells, embryonic stem cells, newly discovered inducible pluripotent stem cells.
7.3.3.1 Adult Chondrocytes
Adult chondrocytes are relatively simple to isolate and cultivate; these cells can be used to directly synthesize a cartilage-specific extracellular matrix [58]. A primary monolayer culture of chondrocytes can express a specific extracellular matrix, such as type II collagen and proteoglycans, which can be maintained for several weeks after passage. However, there is a limited source of adult chondrocytes, which tend to lose their phenotypes after multiple passages and culturing in vitro and to differentiate into fibroblasts, which cannot secrete a cartilage matrix. Consequently, the synthesis and secretion of type I and type III collagen increase, and the adult chondrocytes gradually lose their originally well-differentiated phenotypes, i.e., the tendency to dedifferentiate. The loss of phenotype has limited the large-scale in vitro proliferation of chondrocytes, making it difficult to obtain cartilage tissue with normal function after in vivo transplantation. Adult chondrocytes are usually used in conjunction with a scaffold or cell carrier, the surface features of which are used to maintain the normal matrix-secreting function of the chondrocytes. Autologous chondrocyte transplantation has been successfully carried out in clinical practice, and satisfactory early treatment results have been obtained [59]. Animal experiments have shown that transplanting a chondrocyte/scaffold complex, which has been constructed in vitro, into large rabbit cartilage defects can promote the processes of repair and reconstruction [60]. The emergence and development of a 3D culture technique have enabled an extracellular matrix microenvironment to be simulated in the body. Chondrocytes, which have been cultured in vitro, can maintain a stable phenotype with a well-differentiated state and can even transform dedifferentiated chondrocytes in a monolayer culture into a well-differentiated state.
7.3.3.2 Bone Marrow Mesenchymal Stem Cells
Bone marrow mesenchymal stem cells (MSCs) are precursors to various mesenchymal cells such as osteoblasts, chondroblasts, and bone marrow stromal fibroblasts [61]. MSCs have a multi-directional differentiation potential with a high degree of evolutionary conservation. For over two decades, studies on the growth and differentiation of bone marrow MSCs have shown broad applications for stem cells that have been isolated and cultured from bone marrow by cartilage tissue engineering [62]. Currently, the isolation and application of MSCs is an important research subject in tissue engineering worldwide; experiments have shown that MSCs have a strong in vitro proliferative capacity and can be induced to differentiate into chondrocytes and form cartilage tissue in vivo. MSCs can be easily obtained via a simple bone marrow puncture: A couple dozen millimeters are sufficient to extract the number of cells needed in clinical trials. MSCs can be introduced into cartilage defects by two methods. The first method is the direct transplantation of MSCs into joints. Wakitani et al. were the first to transplant a complex of autologous bone marrow MSCs (which were cultured in vitro) and a type I collagen gel to repair rabbit articular cartilage full-layer defects [63]. The hyaline cartilage formed after only 2 weeks; by week 24, the articular cartilage and the subchondral bone defects were repaired, but the repaired tissue was thinner than the healthy tissue, and there was a gap between the repaired tissue and the healthy cartilage tissue. In the second method, chondrocytes that are induced in vitro or genetically modified chondrocytes are retransplanted into the defect area. Butnariu-Ephrat et al. used a high-density in vitro culture to induce MSCs into chondrocytes, which then formed a chondrocyte/2 % high molecular weight hyaluronic acid complex that was autologously transplanted to repair sheep articular cartilage defects [64]. A hyaline cartilage-like tissue similar to the normal articular cartilage structure formed after only 3 months.
7.3.3.3 Embryonic Stem Cells
Embryonic stem cells have an unlimited proliferative capacity and versatile differentiation; consequently, embryonic stem cells have a higher potential than adult stem cells to become new tissue engineering seed cells [65]. Embryonic stem cells have been successfully induced to differentiate into chondrocytes in vitro and have even been used in attempts to construct cartilage tissue [66]. However, embryonic stem cell lines are difficult to obtain and establish. There are many challenges associated with the use of embryonic stem cell lines including safety, ethical, and immune rejection issues [67]. Therefore, there is currently only limited application of embryonic stem cells in tissue engineering.
7.3.3.4 Induced Pluripotent Stem Cells
Induced pluripotent stem (iPS) cells is a newly developed stem cell technology in which differentiated adult cells (such as skin cells) are introduced into a series of genes (Oct-3, Sox2, c-Myc, Klf4, and Nanog) and are then re-encoded into stem cells with multi-directional differentiation potential [68]. In this technique, isolated autologous adult differentiated cells are first re-encoded into stem cells, which differentiate into specific tissue cells under certain culture conditions. The specific tissue cells are then used for tissue engineering. All of the methods for obtaining induced pluripotent stem cells reported before March 2009 used a virus to transplant various genes into skin cells to promote cell transformation [69]. Both the viral vector and the transplanted gene pose cancer risks, which has greatly limited iPS application. Recently, breakthroughs have continued to be made with the iPS technique, such as bypassing the use of dangerous viral vectors to reduce the number of types of introduced genes and clean up the transplanted genes after the “usage time,” thereby avoiding the various risks introduced by foreign genes [70]. It has been reported that iPS has been successfully induced in chondrocytes [71]. Thus, a series of dangerous or potentially dangerous risks has been circumvented, and we anticipate that the unlimited potential of iPS will be tapped for cartilage damage repair.
7.3.4 Cartilage Tissue Engineering Scaffold
An ideal scaffold is crucial for the successful construction of tissue-engineered cartilage. An ideal scaffold should meet the following criteria: (1) Good biocompatibility, which is required for seed cell adhesion, proliferation, growth, and differentiation; (2) A 3D structure with an optimal porosity of over 90 %; (3) Good biodegradability, with non-toxic degradation products that can be absorbed by the human body; (4) An effective matrix–cell interface for cell adhesion and growth, which, more importantly, can activate cell-specific gene expression and maintain the normal cell phenotype; (5) Plasticity and a prescribed mechanical strength to support new tissue. According to different sources, biologically active materials in tissue engineering can be classified into natural and synthetic materials. Natural biological materials generally have cell signal recognition capabilities; can promote cell adhesion, proliferation, and differentiation; generally have no toxic side effects; and possess good biocompatibility and biodegradability [72]. Polysaccharides and protein materials are commonly used because they are the main components of the extracellular matrix and can effectively simulate the microenvironment necessary for cell growth. Common natural polysaccharide materials include chitosan, chitin, chondroitin sulfate, and hyaluronic acid [73]. The proteins used as biological materials mainly include collagen, gelatin, and fibrin. These materials offer the advantage of carrying considerable biological information that enables cells to produce or maintain various functions [74]. These materials are directly derived from plants and animals and thus have good biocompatibility. The microstructure, the mechanical properties, and the degradation time of synthetic polymer materials can be predesigned and controlled [75]. Currently, poly(lactic-co-glycolic acid) (PLGA), which exhibits good biocompatibility, controlled degradation ability, etc., has been approved by the United States. FDA is a tissue engineering scaffold and is widely used [76]. However, PLGA also has many disadvantages for practical applications, such as insufficient hydrophilicity, weak cell adhesion, and the potential for inflammatory reactions of the acidic degradation products [77]. Composite materials are currently being intensively researched in tissue engineering: Two or more types of biological materials with complementary characteristics are combined in specified proportions following a particular method to produce a 3D material with an optimal structure and properties that compensate for the drawbacks of the individual materials themselves. Continuing advances in molecular biology, material science, and other disciplines have produced new materials such as electroplating chitosan/polyethylene oxide ethylene, fibrin polyurethane, and fiber bacterial cellulose. Experiments have shown that these scaffolds can act as artificial cartilage scaffold [78]. However, an ideal material has not been found thus far, and the search continues for a scaffold with enhanced cell compatibility, a controllable degradation rate, and a prescribed mechanical strength that can be used in current articular cartilage tissue engineering
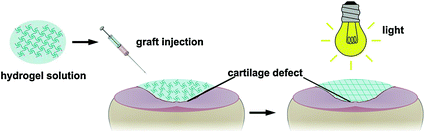
.

Fig. 7.3
The hydrogel solution is injected into the cartilage defect and photopolymerized in situ with light.
7.3.5 GAM Advantages for Cartilage Repair
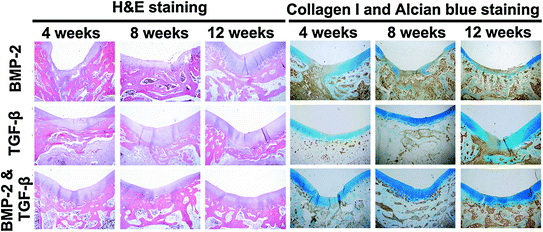
GAMs offer several advantages for cartilage repair. (1) A GAM can be directly applied inside the articular cavity, which prevents excessive degradation of the genetic components by nucleic acid enzymes in the body’s circulatory system; the resulting locally high DNA concentration enhances the transfection efficiency. Local application also avoids ectopic transfection and is therefore safer. (2) The GAM functions as a targeted drug delivery system by directly acting on and targeting the cartilage repair cells; therefore, gene drug delivery into the joint cavity is maximized, and the genetic components are concentrated in the target area to several times or even hundreds of times the concentration of the systemic administration [79]. Huang et al. complexed BMP-4 plasmid and PEI, and the nanocomplex was encapsulated in a PLG scaffold. Researchers observed that this delivery strategy allows gene expression for periods of up to 18 weeks and achieved better therapeutic effect than blank scaffolds in a rat critical-sized defect [79, 80]. Therefore, the interaction between the DNA and the target cells is prolonged, thereby significantly improving the treatment results and reducing the amount of DNA used in the body and the rate of ectopic transfection. A GAM functions efficiently at low toxicity and therefore promotes safety. (3) The articular cartilage tissue cannot easily access large doses of cytokines from the blood circulation system, and it is therefore especially important to maintain a suitable concentration of cytokines in the defect area. In addition to facilitating the adhesion, proliferation, and differentiation of chondrocytes, the genetic components carried by the GAM can be locally expressed to secrete highly active therapeutic agents and promote repair. (4) The cartilage repair process consists of a single stage and does not require the long-term expression of the gene product. The DNA in the GAM has specific release kinetics to meet the requirements of the treatment window of the growth factor, thus avoiding excessive DNA dosing [81]. (5) A GAM may be incorporated into a 3D scaffold to provide filling support for the cartilage defect area; the scaffold is not affected by the range, size, and depth of the defect area and can be cut into any desired shape for direct injection into the articular cavity. Injectable in situ cross-linkable gels are highly desirable clinically as they can be performed using an arthroscopy, a convenient and less invasive procedure (Fig. 7.3). A recent study demonstrated that an alginate hydrogel containing BMP-2 plasmid and MSC could secrete biologically active BMP-2 protein 6 weeks after implantation. The protein levels were effective in inducing osteogenic differentiation as demonstrated by the production of collagen I and osteocalcin [82]. Injectable hydrogels containing plasmid encoding growth factors appear to be a promising new strategy for minimal-invasive delivery of growth factors in cartilage regeneration. (6) A variety of therapeutic genes can be composited together to synergistically affect cartilage defect repair. Our studies using gene therapy for cartilage repair applications have utilized growth factors such as TGF-β and BMP-2, which can promote the regeneration of both cartilage and subchondral bone [57]. From the results of H&E staining and immunohistochemical staining of collagen I and Alcian blue staining in Fig. 7.4, it is observed that the GAMs containing TGF-β or BMP-2 alone showed weakness in the repair of either subchondral bone or cartilage and need more wound healing time. Another study reported a combination of anabolic (IGF-1) and catabolic (IL-1 antagonist) to regulate tissue homeostasis using gene therapy. The catabolic proteins inhibit expression of genes related to catabolic tissue response, while anabolic proteins stimulate matrix production [83]. These studies indicate that multiple gene therapies have great potential in cartilage defect repair applications.