Fig. 4.1
Anoikis activation pathways. Lack of adhesion to the extracellular matrix via integrins (transmembrane proteins consisting of α- and β-subunits) reduces the anti-apoptotic signals Bcl-2 and Bcl-XL. The pro-apoptotic pathways involving increase in pro-apoptotic molecules Bid, Bim, Bad, Puma, and Noxa are therefore not inhibited, leading to an intracellular caspase cascade that causes cytochrome c (cyt-c) in mitochondria to be released and apoptosome assembly in the intrinsic pathway. The extrinsic pathways are activated by extracellular death ligands such as FasL binding to corresponding death receptors Fas. There are two branches of the extrinsic pathway: The first leads to a caspase cascade resulting in the direct proteolysis of target proteins, while the second results in a truncated Bid (t-Bid) that joins the intrinsic pathway to activate cytochrome release from mitochondria. [9] Recreated from Fig. 1 of Ref. [9] with kind permission from John Wiley and Sons (License Number 3125710475964)
There are two branches of the extrinsic pathway, both of which begin with the binding of extracellular death ligands Fas ligands (FasL) and tumor necrosis factor-α (TNF-α) to the cells’ corresponding transmembrane receptors Fas and TNF-α receptor TNFR, which are upregulated upon disengagement from ECM [19, 20]. The morphological change into a rounded cell further causes the accumulation and activation of the mentioned receptors [21, 22]. FasL binds to its receptor present on the cellular membrane, causing death-inducing signaling complex (DISC) to be formed. Subsequently, DISC causes the activation of caspase-8 that activates a caspase cascade involving caspase-3 and caspase-7, causing the proteolysis and ultimately cell death (type I extrinsic apoptosis); caspase-8 also can cleave pro-apoptotic Bid into a form (t-Bid) that encourages apoptosome assembly and cytochrome c release, facilitating cell death in the second type of apoptosis [9].
4.1.2 Biomaterials in Cell Delivery Vehicles
A wide variety of biomaterials, both naturally derived and synthetic, have been used in constructing three-dimensional (3D) cell delivery vehicles or cell-laden scaffolds. These scaffolding systems have to fulfill several basic criteria in order to successfully encapsulate cells in vitro and subsequently deliver the functional cells to the target site in the clinical setting. Most importantly, biomaterials used must be biocompatible, that is, they must possess the ability to perform in vivo without invoking any harmful effects such as immune response [23]. Secondly, they should provide a suitable microenvironment for cells to attach, reside, and proliferate within. Thirdly, biomaterials should be biodegradable with a controlled rate—degradation by-products should not be harmful to cells and the rate should match that of tissue growth. Lastly, as cell delivery vehicles, suitable mechanical integrity for ease of handling ex vivo and similar mechanical properties as surrounding tissues in vivo is critical. Simply put, the cell delivery vehicle should recapitulate both the micro- and macroenvironments of the target implantation site; therefore, the materials utilized in constructing the vehicle are of critical importance.
Biomaterials can either be naturally derived or be synthetically manufactured (Table 4.1), both of which possess respective pros and cons. Naturally derived biomaterials are popular because of their low toxicity, biodegradability, low cost of manufacture, and possession of cell-adhesive moieties. However, it is precisely this ability to interact with cells that also imparts the risk of immune response and disease transfer; strict screening and purification are required. Batch-to-batch variation and therefore uncontrollable mechanical properties and degradation rates are also cause for concern.
Table 4.1
List of popularly used naturally derived and synthetic biomaterials for cell delivery
Naturally derived | Synthetic |
|---|---|
Alginate | Poly(acryl amide) (PAam) |
Agarose | Poly(acrylic acid) (PAA) |
Chitosan | Poly(ethyl glycol) (PEG) |
Chondroitin sulfate | Poly(ethylene oxide) (PEO) |
Collagen | Polylactic acid (PLA): poly-L-lactic acid (PLLA), poly-DL-lactic acid (PDLLA) |
Elastin | Poly(lactide-co-glycolide) (PLGA) |
Fibrin | |
Gelatin | |
Hydroxyapatite | Poly(vinyl alcohol) (PVA) |
Hyaluronic acid | Poly(ε-caprolactone) (PCL) |
Silk fibroin |
Synthetic biomaterials circumvent the need for purification and batch-to-batch variation. Synthetic polymers have predictable and controllable chemical and physical properties, but are more expensive and do not possess cell adhesion moieties. Furthermore, their degradation by-products may be toxic to cells. Therefore, synthetic materials require functionalization to be biocompatible. Popular synthetic biomaterials include poly(ethyl glycol) (PEG), polylactic acid (PLA), and poly(vinyl alcohol) (PVA). PEG is not naturally degradable and cell adhesive, requiring addition of cleavable segments such as polyester units and coatings with cell adhesion moieties.
Often, the best of both worlds—respective advantages of naturally derived and synthetic biomaterials—are combined by researchers in a bid to create an “ideal” biomaterial with the desired properties for the target delivery cell type and the target implantation site [24].
4.2 Engineering Basic Cell Delivery Structures
Cell delivery vehicles (or scaffolds) can be categorized into preformed or injectable systems. Preformed systems are fabricated ex vivo; cells are introduced either during or after the fabrication process of a macroscopic-sized scaffold. The cell-laden scaffolds are then cultured in vitro for a period of time prior to surgical implantation. On the other hand, injectable systems are either in cell-encapsulated microsized scaffold (microcarrier) form or in a cell suspension form, which can be easily injected into the target site, thereby providing a minimally invasive means of implantation. The latter refers to hydrogel solutions that can be gelled in situ under mild conditions, imparting the quality of filling up irregular defects.
In the subsections below, the different fundamental structures and their fabrication techniques, as well as the popular biomaterials used in cell delivery vehicles are discussed. Biomaterials are often engineered to achieve quick and simple polymerization as well as to suit the cell type and the target site; they can be conjugated with polymerizable segments, blended or copolymerized with other materials prior to fabricating the scaffold structures or fabricated with certain orientations. Table 4.2 summarizes and compares the four different basic structures.
Table 4.2
Features, fabrication techniques, and development of basic cell delivery structures
Hydrogel | Fibrous mesh | Sponge | Decellularized scaffold | |
|---|---|---|---|---|
Features ✔: advantages – : disadvantages | ✔ Highly hydrated, mimicking biological tissue | ✔ Fibers mimic ECM fiber architecture in structural tissues (collagen) | ✔ Highly porous | ✔ Provides tissue-specific native ECM and growth factors (niche) by previous residing cells |
✔ Strong mechanical properties, suitable for structural tissues − Small, thin templates for proper diffusion − Limited amount of pores for structural integrity; limited interconnectivity − Irregular pore size − Difficulty in ensuring well-distributed pores − Require removal of toxic components used in fabrication − Time-consuming, labor intensive post-fabrication washing − Require prefabrication before cell seeding | ||||
✔ Good diffusion of nutrients and waste | ✔ Strong mechanical properties, suitable for structural tissues, e.g., bones | |||
✔ Able to retain complex structure and ECM architecture that cannot be easily mimicked (e.g., alveoli of lungs) | ||||
✔ Mild conditions for polymerization (fabrication) | ||||
✔ Highly controllable physical and chemical parameters | ||||
✔ Reversible gelation (responsive to external stimuli, e.g., change in temperature and ionic concentration) | ||||
✔ Biocompatible | ||||
✔ Controllable fiber diameters (nm to μm scale) − Time-consuming, labor intensive − Non-injectable − Require prefabrication before cell seeding | ✔ Xenogeneic and allogeneic sources used without eliciting immune response as ECM components are well conserved among species − Labor intensive, time-consuming as complete removal of cells and nuclear material required − Decellularization protocols may cause structural ECM changes | |||
✔ Typically degradable | ||||
✔ Fast and simple gelation | ||||
✔ Injectable − Weak mechanical properties, suitable for soft tissues only | ||||
Fabrication techniques | • Photoinitiated (conjugate with acrylate groups) • Enzymatic (conjugate with phenolic hydroxyl groups) • Temperature (e.g., agarose, gelatin) • pH (e.g., chitosan) • Ionic (e.g., alginate) • Self-assembly peptides/ amphiphiles | • Electrospinning • Extrusion • Phase separation | • Gas foaming • Particulate leaching/ solvent casting • Freeze-drying • Phase separation • Thermally induced phase separation (TIPS) | • Physical (freeze–thaw, agitation, sonication) • Enzymatic (trypsin, endonucleases) • Chemical (SDS, Triton X-100) |
Modification | • Interpenetrating network (IPN) of two hydrogels [27] | • Coatings [56] • Aligned fibers [58] • Different shapes, e.g., tubes and mats • Zonal organization [55] |
4.2.1 Hydrogels
Hydrogels are a class of insoluble water-swollen polymeric networks formed from crosslinked water-soluble macromers. Usually, cells are mixed into the hydrogel precursor solution prior to gelation, hence achieving a homogeneous cell-laden hydrogel (Fig. 4.2a). Advantages of hydrogels are as follows: the highly hydrated environment and good diffusion of nutrients and waste as well as the mimicking of most tissues. Many hydrogels offer the advantage of being injectable: In other words, they can be quickly gelled in situ under mild physiological conditions, thereby allowing molding into irregularly sized defects via a minimally invasive means of implantation. These properties have thus made hydrogels popular for cell and drug delivery. Through altering the porosity and crosslinking density, mechanical properties of hydrogels can be adjusted to suit the targeted cell type and site of implantation. Mechanical strength, however, is usually low compared to the meshes and sponges, and hence, hydrogels are only suitable for soft tissues such as cartilage. Furthermore, as hydrogels are highly hydrophilic, cells typically exhibit a rounded morphology when encapsulated within [25]. While this makes hydrogels suitable for the delivery of non-ADCs, successful delivery of ADCs using hydrogels would require modifications to include cell adhesion moieties within the hydrogels for ADCs to adhere onto.
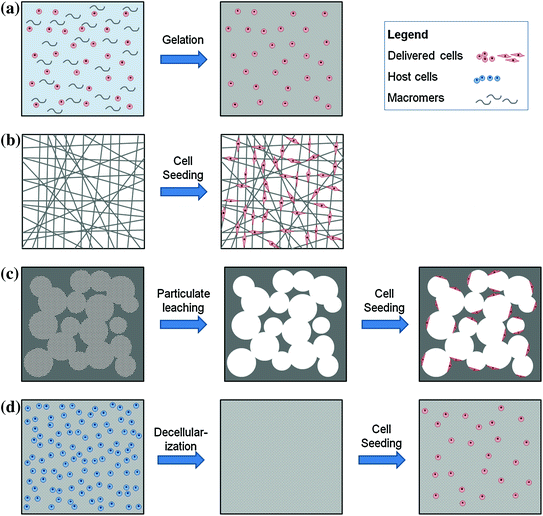

Fig. 4.2
Basic cell delivery structures. (a) Hydrogel formation from homogenous cell suspension in macromer solution; (b) fibrous mesh fabrication via electrospinning and subsequent cell seeding and attachment onto fibers; (c) sponge fabrication via particulate leaching by firstly forming a solid encapsulating particulates (porogens), leaching of particulates to leave behind pores, and finally seeding cells that attach to walls of pores; and (d) decellularized scaffolds fabrication by firstly isolating biological tissues and organs from allogeneic or xenogeneic sources, applying decellularization techniques to remove previous host cells without breaking down ECM structure and finally seeding therapeutic cells onto decellularized ECM scaffold
Hydrogel formation (gelation) is attained via physical or chemical crosslinking mechanisms. Under suitable conditions, a solution consisting of macromers or unreacted monomers with crosslinking agents is converted into an insoluble 3D network upon gelation. Physically crosslinked gels are linked via ionic or hydrogen bonds or hydrophobic interactions, forming a gel upon a change in environmental conditions, e.g., temperature, pH, and ionic concentration. Temperature-responsive hydrogels such as agarose and gelatin have reversible properties. Alginate, derived from brown algae, is an ionic polysaccharide that is crosslinked upon presence of divalent cations such as calcium. These are usually reversible crosslinking reactions—upon reversal of conditions, the gel reverts to a solution form. Chemically crosslinked hydrogels are linked via covalent bonds—radical initiators activate crosslinking agents that link monomers to a certain critical density that converts the precursor solution form into a gel form. An example would be Photoinitiated polymerization using ultraviolet or visible light, which is a popular crosslinking technology because it offers injectability [26].
Photoinitiated crosslinking is a popular technique using ultraviolet (UV) radiation, whereby gelation is achieved at physiological temperature and pH under light exposure. Macromers such as PEG are firstly conjugated with acrylate groups and subsequently mixed with a small amount of photoinitiators and cells prior to exposure to light for gelling. Depending on the mechanical strength required which is dependent on crosslinking density, the density of conjugated acrylate groups or the duration of light exposure can be varied. Alginate was conjugated with methacrylate through an esterification reaction to become photoinitiated crosslinkable [27]. To provide the cell adhesion moieties, the alginate–methacrylate precursor was mixed with temperature-responsive collagen solution and gelled by firstly increasing the temperature to 37 °C for collagen gelling and then exposing to UV light for alginate crosslinking, thereby fabricating an interpenetrating network (IPN) of two different hydrogels. This IPN hydrogel was reported to have a denser network and hence had superior mechanical properties than photoinitiated crosslinked alginate gel (control). Furthermore, it was able to support murine preosteoblasts MC3T3-E1 (as an ADC type) for bone defect repair in terms of extensive cell spreading morphology, high proliferative rates, and maintenance of osteogenic gene expressions as compared to the control.
Enzymatically crosslinked gels have recently been gaining popularity due to its injectability and gelation under physiological conditions. A gel can be formed in situ by mixing the phenolic hydroxyl-conjugated macromer solution with hydrogen peroxide (H2O2) and horseradish peroxidase (HRP) enzyme in the target defect site. This peroxidase-catalyzed system has been applied in various polysaccharides including chitosan, hyaluronic acid, alginate, and dextran [28–32]. Notably, gelatin type A was modified to possess tyramine (phenolic hydroxyl groups), which was subsequently used for the delivery of osteoblasts [33]. Murine preosteoblasts MC3T3 cells were mixed in the tyramine-conjugated gelatin solution and gelled upon the addition of HRP. The osteoblasts were observed to have a spreading morphology even though osteogenic gene and protein analyses were not significant. Inclusion of bioactive molecules that can promote the osteogenic phenotype, e.g., bone morphogenetic proteins (BMPs) and fibroblast growth factors (FGFs), may be beneficial [34, 35]. Recently, Mathieu et al. injected a suspension of mesenchymal stem cells (MSCs) and a pH-responsive hydrogel into infarcted myocardia of rats and observed an increased healing rate and functionality with little fibrosis [36]. The silane-grafted hydroxypropyl methylcellulose (Si-HPMC) gels upon a decrease in pH levels to the physiological pH 7–7.4, due to the condensation of silane groups.
Self-assembling peptides spontaneously assemble themselves into a stable macroscopic nanofibrous network via non-covalent interactions such as hydrogen bonding, hydrophobic and Van der Waals forces [37]. Being amphiphilic, i.e., containing both hydrophilic (polar) and hydrophobic (non-polar) amino acid residues, the peptides undergo self-assembly to undertake the most stable structure as a nanofiber in a polar environment (salt solution or cell culture media)—non-polar residues are orientated into the fiber center while exposing the polar residues to the environment. This phenomenon is ubiquitous in nature, e.g., phospholipids in forming micelles and plasma membranes. As the self-assembled scaffolds are simple and form under physiological conditions, have a fiber diameter of approximately 10 nm and pore diameter of 5–200 nm and mimic ECM structure, are highly hydrated, and can be functionalized with the addition of cell adhesion moieties, they have been extensively utilized as a tissue engineering scaffold [37, 38].
For example, to impart the cell adhesion capability to the self-assembled nanofibrous hydrogel, cell adhesion moieties such as arginine–glycine–aspartic acid (RGD) sequences can be conjugated to the peptides prior to assembly [39–41]. Zhou et al. [39] designed a self-assembled hydrogel based on two simple peptides, one of which possesses the RGD sequence. The sequence not only provides the hydrogel with cell-adhesive properties on the surface of the nanofibers, but it also influences the mechanical properties of the hydrogel. Human dermal fibroblasts encapsulated within the hydrogel were able to adhere through RGD-specific binding and exhibited a spreading morphology as ADCs do. Webber et al. [41] also demonstrated the potential of self-assembled hydrogels made up of RGD peptides in delivering bone marrow mononuclear cells (BMNCs) into mice. BMNCs were able to adhere and proliferate in the tested hydrogel in vitro, enhancing the cells’ numbers by more than 5-folds in 5 days, whereas non-RGD-containing nanofibrous hydrogel control had no significant increase in cell number during the same time period. Furthermore, gene expression studies validated that BMNC phenotype was maintained in vitro; in vivo experiments also confirmed that BMNCs were successfully delivered subcutaneously with maintenance of high viability, albeit with a mild tissue reaction to the cell delivery material.
Gong et al. [42] made use of a naturally occurring phenomenon involving non-ADCs and the non-cell-adhesive property of hydrogel to develop a dense tissue construct for cartilage tissue engineering. A rapid and dense outgrowth of cells and ECM secretion at gel–medium boundaries, termed “edge flourish,” was exploited in a microcavitary gel (MCG)—chondrocytes and gelatin microspheres were coencapsulated in temperature-responsive agarose hydrogel; upon raising the temperature to 37 °C, gelatin melts to leave behind microcavities within the hydrogel bulk. The strategy was named phase transfer cell culture (PTCC) to connote the dynamic culture of cells on the boundaries of two phases. Chondrocytes were then observed to infiltrate and fill up the cavities. By inducing cavities to increase the amount of gel–medium boundaries throughout the hydrogel bulk, not only was diffusion of nutrients and waste improved, a higher proliferation rate and hyaline cartilage-specific ECM secretion was observed [42]. In another study involving a temperature-responsive hybrid hydrogel of naturally derived heparin and synthetic PEG diacrylate (PEGDA) was shown to support primary hepatocyte spheroids more greatly than pure PEG-based hydrogels, as concluded from the high cell viability and albumin and urea secretion [24]. The heparin–PEG hydrogel gels at 37 °C within a short duration of 10 min under physiological conditions and was also able to retain hepatocyte growth factor (HGF) and maintain its bioactivity; this hydrogel is therefore envisaged to be a potential stem cell delivery vehicle with differentiation cues incorporated within.
A relatively new synthetic saccharide–peptide hydrogel has been designed and demonstrated to be highly supportive of non-ADC chondrocytes [43] and pancreatic islets [44]. Being composed of naturally derived monomers saccharides and peptides, the intrinsic advantages of biodegradability, non-toxicity, and low cost are combined with the controllable and predictable properties of synthetic biomaterials. The copolymer backbone is covalently crosslinked via Michael-type addition mechanism and subsequently functionalized with tyrosine amino acids which, in chondrocyte studies, supported extensive ECM secretion and higher mechanical strength [43]. In another study involving pancreatic islet delivery into rat models using the same gel, the diabetic condition was significantly reversed alongside the high insulin secretion by the functional islets and lack of detectable immune response, as compared to transplanted unencapsulated islets [44].
4.2.2 Fibrous Meshes
Fibrous meshes are a body of individual nanoscale fibers that have been spun into a 3D network. Its structural similarity to native ECM network, high surface area to volume ratio, porosity, tunable mechanical and degradation properties, and controllable fiber diameter [45] makes fibrous meshes as suitable cell delivery vehicle, especially for ADCs (Fig. 4.2b). Electrospinning is a common technique for fabricating fibrous meshes. The droplet of polymer solution forms a thin stream upon passing through an electric field and is collected as a mesh of fibers upon the evaporation of solvent in the polymer. By modifying the collector and electric field, fiber orientation [46] as well as scaffold shape and size [47–49] can be varied. For example, tubular scaffolds were fabricated using a rotating rod collector [50]; tubular structures with longitudinally aligned nanofibers were fabricated using two extra parallel electrodes [47]. However, the difficulty in cell penetration into a dense scaffold is a shortfall [51]. Naturally derived materials including elastin [52], sulfated silk fibroin [53], collagen [54], and synthetic materials PCL [46, 55] and PLA [56] have been used in fabricating electrospun scaffolds.
While many previous studies utilized simple randomly aligned fibrous meshes, second-generation fibrous meshes with aligned fibers and multilayered structures have been engineered to better mimic the complex native ECM structure. Since fiber orientation can modulate cell phenotype and guide cell growth, many studies have deliberately aligned fibers for the culture of muscle cells and neurons. For example, smooth muscle cells (SMCs) cultured on longitudinal poly(L-lactide-co-ε-caprolactone) P(LLA-CL) 75:25 fibers had a contractile phenotype similar to those in physiological conditions [57]. Another research group demonstrated the effect of aligned PCL/collagen fibrous meshes on skeletal muscle cells [58]. The skeletal muscle cells were stretched along the fibers, exhibited high viability and functionality with formation of myotubes observed.
In a bid to restore the neural retina through cell-based therapy, biodegradable PLA was utilized in fabricating a scaffold of radially aligned nanofibers mimicking the ECM architecture in the retina, for the culture of retinal ganglion cells (neurons) [56]. The scaffold was then immersed in laminin to coat the fibers as neurons reside in a laminin-rich ECM [59]. Axons of the cells, as a type of ADCs, were observed to be of higher viability and were mostly aligned along the fibers, as opposed to control tissue culture plates and randomly aligned electrospun scaffolds. Cells cultured in the aligned scaffolds maintained electrophysiological functionality and exhibited similar radial patterns as axon bundles of the native retina. Furthermore, at higher densities, the cells were observed to form axon bundles in vivo. This cell delivery vehicle for the treatment of degenerated retina, e.g., due to glaucoma, is an important first step in delivering aligned functional nerve cells and can be developed further for delivering other nerve cells, especially for the repair of the central nervous system, since nerve cells have poor self-repair capability.
Homogeneous fibrous meshes have been widely used as ECM substitutes, but recently the paradigm shifted toward creating structures more similar to native tissue. For example, the zonal organization of articular cartilage was mimicked using PCL fibers of varying orientations to match the mechanical properties of each zone: The topmost superficial layer as a lubricating surface was made of aligned 1-μm-thick fibers; the middle layer was composed of random 1-μm fibers; and the deep layer was composed of random and thicker fibers of 5 μm diameter, mimicking the architecture of collagen fibrils in native articular cartilage tissue [55]. Chondrocyte culture in the triple-layered electrospun scaffold was done in vitro for 5 weeks; analyses of mechanical properties and quality of engineered cartilage revealed close similarity with native cartilage tissue. In another study, McClure et al. [60] designed a triple-layered electrospun scaffold with layer-specific compositions of PCL, elastin, and collagen fibers to mimic the structural architecture of arteries, namely the intima, media, and adventitia layers; the engineered construct was mechanically tested acellularly and demonstrated similar modulus and compliance values to those of native porcine femoral arteries. The potential of this artery-mimicking electrospun scaffold, however, would be better reflected with the culture of vascular cells—vascular endothelial cells and SMCs.
4.2.3 Sponges
Also known as porous scaffolds, sponges are made using gas foaming, particulate leaching, and freeze-drying techniques. The success of porous scaffolds as a cell delivery vehicle depends on its porosity, pore size, and pore interconnectivity—the pores provide surface area for cells to adhere on, while interconnectivity dictates the cell penetration as well as diffusion of nutrients and waste [25]. Pore size is critical: Small pores will impede cell penetration and proper diffusion, while larger pores may not be sensed by cells to be a 3D environment. Hence, engineering a cell delivery capable sponge requires consideration of pore design parameters.
Gas foaming involves the saturation of high-pressure gas (usually carbon dioxide) into the polymer particles; upon rapid decrease in pressure, gas bubbles nucleate and expand and the polymer fuses to form a continuous porous scaffold [61]. This method is quick and simple and allows the control of pore size by varying pressure without the use of high temperatures or harsh organic solvents to create the pores. Recently, PDLLA/PEG copolymer sponges were fabricated by gas foaming, achieving 84 % average porosity [62]. PDLLA possesses superior mechanical properties and biodegradability but is hydrophobic and therefore unsuitable for cell encapsulation. Hence, by copolymerizing with hydrophilic PEG, the advantageous properties of each material are combined. Although no cell-seeding studies were done on this sponge, this is a classic example of exploiting the advantages of two different materials by copolymerization, and with further optimization, the sponge is expected to work well as a cell delivery vehicle. Zhou et al. [63] combined foaming and blending of two immiscible polymers to create a more controllable, open, and porous scaffold with higher interconnectivity between pores. Firstly, two polymers PLA and polystyrene were blended in equal weight ratios together and molded by compression prior to carbon dioxide foaming, achieving a porous scaffold of PLA and polystyrene with tunable pore sizes. Then, polystyrene was removed via immersion of scaffold in cyclohexane, creating a PLA scaffold with much higher interconnectivity. After washing, osteoblasts were seeded onto the scaffold and compared against unfoamed control; although both scaffolds showed proper cell adhesion and spreading morphology of osteoblasts, the foamed sample had significantly higher number of cells, thereby reiterating the importance of porosity.
Particulate leaching using salt particles is an alternative method to create porous scaffolds (Fig. 4.2c) by mixing salt crystals (typically sodium chloride NaCl) into a polymer solution and then inducing the crosslinking to form a scaffold with salt particles entrapped within. Upon immersing in water, the salt dissolves and is removed from the scaffold, leaving behind empty spaces inside the scaffold. Sponges of silk fibroin/hyaluronic acid blends for cartilage tissue engineering were fabricated using this method [64]. Silk fibroin/PDLLA sponges were fabricated by NaCl particulate leaching; the silk fibroin was incorporated to increase the vascularization potential of the PDLLA sponge for bone tissue engineering, since silk fibroin is known to support the adhesion and growth of endothelial cells [65]. In this study, human umbilical vein endothelial cells (HUVECs) were shown to be properly adhered onto the silk fibroin/PDLLA sponges with high viability and proliferation while maintaining the cell phenotype in vitro; in vivo studies showed quicker vascularization and integration with the surrounding tissue.
PLA/PCL (70:30) sponges were also fabricated using NaCl particulate leaching and studied for the delivery of myoblasts for skeletal muscle repair [66]. Myoblasts were not only able to adhere and grow on the sponges, but they also differentiated and fused with each other into myotube structures with expression of skeletal muscle-specific genes and proteins, both in vitro and in vivo. Coupled with the biodegradability and biocompatibility of the materials, the PLA/PCL sponge exhibits great potential for skeletal muscle repair.
4.2.4 Decellularized Scaffolds
Biological tissues and organs comprised of ECM network and residing native cells can be extracted and then decellularized (removal of cells), leaving behind an intact ECM scaffold on which cells can be reseeded prior to implantation (Fig. 4.2d) [67]. Decellularized scaffolds provide a most suitable natural microenvironment as tissue- and organ-specific ECM cues produced and modeled by the previous residing cells can guide the newly seeded cells. Complete decellularization is important to remove the immunogenicity caused by previous cells’ antigens, since the host tissue is of xenogeneic and allogeneic origin. This can be achieved by chemical (SDS, Triton X-100) [68], physical (freeze–thaw cycles), or enzymatic (trypsin, endonucleases) means [69]. Indeed, the excellent biocompatibility and lack of immunogenicity of decellularized scaffolds have been recognized and approved by FDA for bone [68], skin [70], and heart valve replacements [71] as acellular scaffolds, whereby host cells are allowed to penetrate and regrow tissue. Other decellularized scaffolds currently being studied and characterized include skin [72, 73], larynx [74], lung [75, 76], blood vessels [77, 78]. To accelerate the healing process, cells can be seeded within the decellularized scaffold and cultured in vitro, thereby generating a replacement tissue or organ ready for implantation [79, 80].
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


