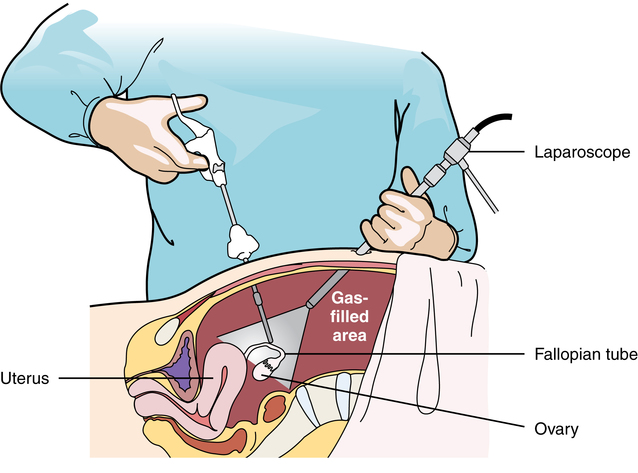
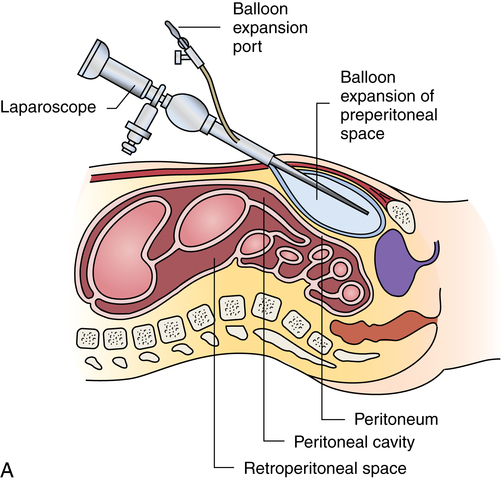
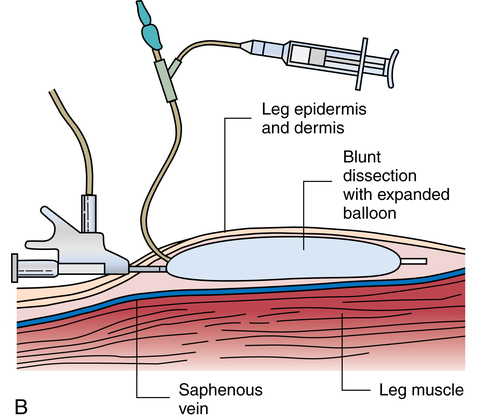
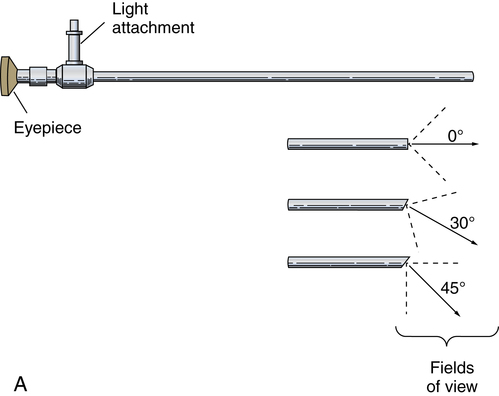
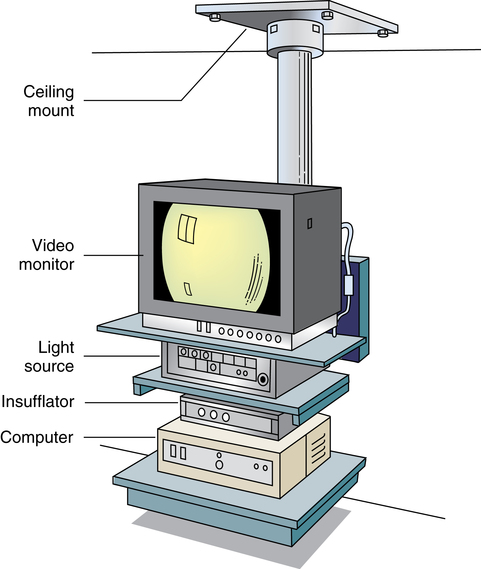
Chapter 32 After studying this chapter, the learner will be able to: • Describe the difference between rigid and flexible endoscopy. • List the eight essential elements necessary for all endoscopy. • Identify three potential hazards of puncture endoscopy. • List three considerations for patient safety during endoscopy. Endoscopic technology, regardless of whether it is performed with a rigid scope or a flexible scope, has eight elements in common (Box 32-1): These essentials of endoscopy are described in more detail as follows: 1. Natural orifice or functional stoma a. Oral, nasal, vaginal, anal, urethral. Some rigid scopes have an obturator (a blunt-tipped rod placed through the lumen) to permit smooth insertion of the instrument, such as into the anus. b. Natural Orifice Transluminal Endoscopic Surgery (NOTES). This is one of the newest forms of incisionless surgery. The surgeons using this method voice concerns for better optics and instrumentation. Currently, the surgery is performed by entering the mouth, vagina, or anus. The endoscope is passed into the passage and traversed to a point where an incision can be made into an organ wall to access a structure within the peritoneum. Target structures include the stomach (obesity procedures and fundoplication), adjacent structures, and appendectomy. Research is under way for diaphragmatic pacing wires, pancreatic biopsy, and peg tube revision. The surgeon can enter the mouth and pass the endoscope into the stomach where an incision is made through the gastric wall. The working instruments are used to excise the gallbladder and remove it from the body through the mouth.15 One point of concern is the closure of the gastrotomy and the potential for bleeding and leakage. For gynecologic procedures the endoscope can be inserted through the vaginal wall to perform tubal or ovarian procedures. Women with previous hysterectomies may have adhesions and may not be candidates for a transvaginal approach to the peritoneal cavity. Previous surgery of any kind may be a problem for NOTES endoscopists. Other procedures can be performed transrectally, but there are concerns for bowel contents entering the peritoneal cavity. Research is being done on the feasibility of transesophageal NOTES to enter the thoracic cavity without an external incision. c. Ear canal: Small specula are used to perform procedures within the ear. 2. Puncture or incision: Placement of the initial devices to introduce the expansion medium for creation of the working space requires interruption of the body’s intact surface. Some surgeons use preemptive analgesia with a local anesthetic injection at the port sites at the start of the laparoscopic surgical procedure. Some surgeons inject local anesthetics at the end of the case. a. Multiple sites based on the type of cavity or space and the location of landmarks and blood supply. (1) Open method: An incision is made into the skin and a 5- to 10-mm blunt Hasson trocar and sheath are placed into the cavity or space. Expansion medium can be placed through a port into the cavity or space. This procedure may be used for the patient who has many adhesions or has had multiple previous surgeries. (2) Closed method: A tiny nick is incised into the skin, and a Veress spring-loaded needle is placed through the skin into the peritoneal cavity for the delivery of an expansion medium such as gas. b. Access devices for insufflation and creation of a working space. (1) Veress needle: A tiny 1- to 2-mm nick is made with the scalpel at the inferior edge of the umbilicus. The Veress needle is inserted by blind puncture percutaneously (through the skin) into the abdominal cavity. This needle has an exterior sharp bevel and an interior spring-loaded ball-tip obturator that retracts as it passes through tissue. The valve key on the top should be closed for insertion. As the sharp bevel passes through the tissue layers, the rounded obturator snaps forward to guard against inadvertent punctures of nontarget tissue. Slight popping noises are made by the needle and spring mechanism as the needle passes through each layer. The last sound is the peritoneal perforation. Some surgeons test intraperitoneal placement of the Veress needle by placing a drop of saline onto the Luer-Lok and opening the valve key. The saline is sucked into the aperture of the needle by the negative force of the closed peritoneal cavity. The CO2 tubing can be connected for insufflation of a working space when the surgeon is reasonably assured that the Veress needle tip is in the correct space. The gas flow is started at 1 L/min and increased to 2 L/min. The intraperitoneal pressure should remain at 15 mm Hg for the average adult patient. Methods of placing the Veress needle into the peritoneal cavity include: (a) One-hand placement: The surgeon grasps and lifts the lower abdominal (hypogastric) segment midway between the umbilicus and the superior margin of the pubis. As the abdomen is raised, the surgeon directs the Veress needle through the tissue layers until the peritoneal cavity is entered. (b) Two-hand placement: The surgeon and the first assistant grasp the abdominal tissue on either side of the umbilicus and lift. Some surgeons use Allis forceps or perforating towel clips to grasp the sides of the umbilicus. The surgeon introduces the Veress needle through the abdominal wall into the peritoneal cavity. (2) Trocar and sheath (sleeve): A trocar consists of a sharp or blunt obturator and a sheath or sleeve. Some sheaths have ports with key valves for instillation of fluids or gases. The trocars are inserted with the key valve closed to prevent evacuation of the expansion medium used to create the working space. The primary access portal will be the appropriate size to accommodate the laparoscope. (i) Pyramidal or conical tip: Describes the configuration of the tip. (ii) Shielded: A spring-loaded shield drops down and protects the sharp tip of the trocar when it is not actively cutting through tissue. Studies have shown that this mechanism does not necessarily reduce the incidence of iatrogenic trocar-induced punctures. (b) Blunt trocar: The trocar tip is rounded. The blunt trocar and sheath (Hasson) are introduced through a small infraumbilical incision in the open technique. Silk traction sutures are placed at the level of the fascia and brought out through the skin. The sheath has graduated threads that permit the device to create a seal when placed through the abdominal layers. The traction sutures are tied to the sheath to stabilize and seal the pneumoperitoneum. Insufflation for creation of a working space is performed through the key valve. A Veress needle is not used. Another style of blunt trocar has a distal balloon that is inflated to anchor the trocar in place. It is placed using the open technique. A small foam collar seals the external portion of the incision. (c) Dilating sleeve: The initial trocar/obturator and primary sleeve are small diameter (between 1.9 and 2.1 mm) and are introduced through a small nick in the inferior aspect of the umbilicus. The trocar is rigid, and the sheath is a flexible plastic meshwork with a stopcock for insufflation of the pneumoperitoneum. As the initial trocar is removed, larger-diameter instrumentation can be introduced through the radially dilating sleeve, causing it to dilate or “step up.” The texture of the sleeve expands to accommodate a variety of diameters, such as 5-, 7-, 10-, 11-, and 12 mm instrumentation. The skin puncture stretches and forms a seal around the access portal, anchoring it in position. The fascial puncture can be stretched to accommodate 12-mm instrumentation. The wounds measure around 4 mm when the sleeve is withdrawn and rarely need closure with suture. c. Secondary insertion of accessory trocars for manipulation within the working space can be inserted under direct vision after the laparoscope is inserted into the primary port. Additional trocars and sleeves may be used in a triangular position around the umbilicus or primary trocar. Instrumentation for manipulation and capture is placed though these ports. d. Single Port Laparoscopy (Single Incision Laparoscopy [SILs], Single Port Access [SPA], One Port Umbilical Surgery [OPUS}, and Laparoendoscopic Single Site [LESS]): One periumbilical incision is created and a semiflexible port platform with 3 to 4 lumens is inserted. Insufflation is performed through accessory tubing attached to the device.12 The laparoscope and instrumentation are used through the lumens in the multilumen platform (Fig. 32-1). The single site multilumen platform can be used in robotic applications.5,13 a. Abdominal lift device for creation of a gasless, tent-shaped working space. No expansion media used. The working space is somewhat smaller than that created from gas insufflation. The bowel is not compressed and may be distended. Visualization is affected by the small working space. Care is taken when placing the abdominal lift hooks so as not to snare the omentum or bowel. The lift devices are sometimes difficult to remove without ensnaring abdominal contents. Trocar insertion is sometimes difficult because there is no back pressure or resistance to the force of placement. Blind puncture is not commonly used. An expandable balloon can be used to bluntly dissect and separate the abdominal wall from the abdominal contents for instrumentation placement. It takes some of the pressure off the actual lift device as it pulls upward on the abdominal wall. (1) Linear lift requires a frame such as an arc above the patient that attaches to a cable system placed through the patient’s abdominal wall. The shape of the working space may be irregular. (2) Planar lift uses a corkscrew-like hook or a fanlike retractor that is placed through the abdominal wall and suspended from an articulating arm attached to a frame on the operating bed. The shape of the working space is more trapezoid than domed like a pneumoperitoneum. b. Physiologic muscularity or structural framework, such as cartilage in the trachea and bronchus for upper aerodigestive flexible or rigid endoscopy. c. Speculum support between muscular layers such as the vaginal speculum or anoscope. a. Fluid: The bladder, uterus, and joint spaces can be expanded with fluid to create a working space. b. Gas: The peritoneal cavity is filled with CO2 gas, creating a gas lake called a pneumoperitoneum, before a laparoscope is inserted into the primary trocar through the abdominal wall. The pneumoperitoneum expands the abdomen and provides a working space (Fig. 32-2). With the tip of a #10 or 11 scalpel blade, a small incision (1-2 mm) is made into the abdominal wall at the infraumbilical margin. The spring-loaded 14-gauge Veress needle measuring 70 to 120 cm (average) to 150 cm (obese) is inserted through the tiny incision at the thinnest portion of the abdominal wall. Great care is taken when a Veress needle is used for blind puncture. The major vessels of the abdomen are at risk for injury.11 Some surgeons will check for entrance into the peritoneal cavity with a syringe. Some surgeons will aspirate to check for blood or bowel contents, and others may use a saline-filled syringe to introduce a few mililiters into the opening of the Veress to check for obstruction of the needle. The CO2 tubing is attached to the Veress and the gas is delivered by an insufflator, which is a specially designed machine that allows a metered flow of gas at a controlled pressure of between 12 and 18 mm Hg.16 Pressure gauges on the machine will indicate if the flow is commencing appropriately. The gas passes through sterile disposable tubing with an inline, single-use hydrophobic filter that prevents particulate matter and condensation from the gas tank from entering the abdominal cavity. The insufflation tubing filters particulate as small as 0.2 micron. The insufflator should be positioned higher than the patient’s abdomen for maximum performance. If the cylinder is placed below the level of the patient, the pressure gradient on the patient’s side increases, causing a backflow of biologic contaminants into the insufflator. As a result, the internal aspect of the insufflation machine can become biologically contaminated. This can be the source of cross-contamination between patients. The CO2 gas itself is not sterile and is delivered to the body cold, around 23° C when not passed through a heating and humidifying element. Studies have shown less hypothermia in patients when the gas is delivered at temperatures between 86° F and 95° (30° and 30.5° C). The patient is at risk for hypothermia if core body temperature is less than 36° C. Studies have shown a decrease of 32° F (0.3° C) body temperature for each 50 L of CO2 cycled through the patient. Once the pneumoperitoneum is established, a 10- or 12-mm trocar is inserted and the insufflation tubing is attached to a port on the side of the trocar sleeve. The average adult can accommodate 5 to 6 L of CO2 in the peritoneal cavity to create the working space. A secondary 5- or 7-mm trocar with a gas port will be inserted. c. Air: Ambient room air can be delivered into a natural body orifice by a hand pump bulb or machine to expand the lumen of the bowel for sigmoidoscopy or colonoscopy. d. Balloon expansion between tissue planes: A balloon device can be inserted between tissue layers and expanded with saline to separate and bluntly open the preperitoneal plane of dissection. After blunt dissection and expansion, the balloon is deflated and withdrawn. The space is then insufflated. This is useful for extraperitoneal laparoscopic hernia repair procedures. Saphenous vein harvests and spinal surgery can be performed using this method (Fig. 32-3). 1. Fiberoptic light: With fiberoptic lighting, an intense cool light illuminates body cavities, including those that cannot be seen with other light sources. Light is conducted through a bundle of thousands of coated glass fibers encased in a plastic sheath attached to a light source generator. Each fiber is drawn from optical glass into a strand 10 to 70 mm in diameter that is coated to minimize loss of light by reflection. Light entering one end of the fiber is transmitted by refraction through its entire length. The light produced through the bundle of fibers is nonglaring and evenly distributed on the area to be visualized. Although it is of high intensity, the light is cool. A minimal rise of temperature in the tissues exposed to it may occur. Although the light inside the patient is cool, the cable should not be allowed to lie on the drapes because it is a potential source of ignition. Both ends of the fiberoptic cable must have the correct fittings to attach to the lamp and to the endoscope or removable light carrier. The diameter of the cable varies from 2 to 5.5 mm to be compatible with the aperture for the light source. Cable lengths also vary from 6 feet (180 cm) to 9 feet (275 cm), so the projection lamp can be positioned at a distance from a sterile field. Before use, the fiberoptic cable should be checked for damage. One end of the cable is held toward a low-power light, such as the overhead operating light. With a magnifying glass, the opposite end is examined. Broken fibers in the cable will appear as dark spots like pepper, even to the naked eye. The cable should be replaced if more than 20% of the area appears dark. Newer light cables have a clear sheath for easier visualization of broken fibers. A xenon, quartz-halogen, mercury, or halide light-bulb provides an intense light source for transmission of light through a fiberoptic bundle to the distal end of the scope. Usually a portable, compact, self-contained unit, the light has an intensity that may be regulated from 400 foot-candles to as much as 5200 foot-candles, and up to 5500 Kelvin daylight in some illuminators. a. Incandescent bulbs screw into the fitting either at the end of a removable light carrier or at the end of a built-in lens system. Electric current is conducted through a single-filament wire to illuminate the tiny incandescent lightbulb. Very few instruments use this lighting system. Older-model rigid laryngoscopes are an example. b. Indirect lighting is used with specula, such as vaginal, nasal, or anal. 1. Scope: The length of the scope varies but is appropriate to reach the desired structure. The surgeon views anatomic structures through a telescopic lens. The diameter of the scope varies from the 1.7-mm needle fetoscope to the 5 mm or less of the arthroscope to the 10 to 12 mm of a laparoscope. a. Rigid: A rigid scope is a telescopic rod-lens system that permits viewing in a variety of directions, such as a cystoscope. Laparoscopes are made in two styles—diagnostic and operative. The diagnostic laparoscope is a single telescopic rod-lens with no accessory ports (Fig. 32-4, A). The operating laparoscope has the rod-lens system with one working channel (Fig. 32-4, B). Operating laparoscopes have been in use since the 1970s and were first to use an umbilical single incision approach. Gynecologic surgeons used the operating laparoscope for tubal ligation and exploratory procedures. Pelviscopy was commonly performed by gynecologic surgeons for pelvic surgical procedures such as morselization of fibroids. The reduction in the diameter of laparoscopic instrumentation and increased selection of manipulative tools led to disuse of the larger sized pelviscopic trocars and scopes. Rigid telescopes are metal and use fiberoptic illumination. Disposable models are available. The rigid telescope used in laparoscopy is placed into the body through the trocar sheath. b. Rigid hollow: Disposable plastic sigmoidoscopes, anoscopes, and otoscopes are available. Illumination is from a separate light carrier rod. Rigid bronchoscopes have additional side ports for administration of oxygen or anesthetics. c. Flexible: Flexible scopes have a directional adjuster dial that contours the lensed tip into and around anatomic curvatures to permit visualization of all surfaces of the wall of a hollow structure, such as the upper and lower gastrointestinal tract (Fig. 32-5). The user can look directly into the eyepiece, or an attachment can be placed over the eyepiece to project the image onto a video screen. Flexible endoscopes have working channels that permit the manipulation of flexible biopsy forceps and laser fibers. A separate suction channel is on the opposite side. Two trumpet valves located near the eyepiece control suction and insufflation with air to create a viewing and working space. The covering of flexible endoscopes is made of silicone-plastic material and is usually latex-free. 2. Camera: A camera can be mounted to the eyepiece of the endoscope. Some cameras are used sterile, and others are high-level disinfected and covered with a sterile camera drape. The camera consists of a coupler that attaches to the eyepiece of the endoscope, a camera head, a connecting cable, and a connector to attach the camera to the camera controller box (Fig. 32-6). a. Video: Endoscopes may be attached to a still or video camera so that organs or lesions can be photographed during a procedure. Video cameras with recorder/player/printer equipment require high-powered light sources for photographic applications and video documentation. By viewing high-resolution monitor(s), the endoscopist can manipulate instruments for diagnostic examination or to perform a therapeutic procedure. Video documentation can be obtained by recording the examination or procedure on videotape or to a CD. Microcomputer imaging systems with a printer can reproduce these images into still photographs. This equipment is either kept on a mobile cart or permanently placed in ceiling-mounted articulated booms (Fig. 32-7). b. Monitor: More than one video monitor is recommended for ease of viewing the image transmitted by the endoscope by the entire team. Placement of the monitors in the direct line of vision of the surgeon and the first assistant eliminates the need to turn the head away from the field and prevents stiff neck and fatigue. Newer flat-panel monitors can be suspended from the ceiling on articulated arms that permit the user to place the viewing plane in a position of function. c. Wireless capsule endoscopy: An endoscopic camera can be ingested to visualize the small bowel. The camera weighs around 4 g and measures 11 mm—about the size of a jellybean. The endoscopic capsule contains four light-emitting diodes (LEDs) and a color camera. It travels by peristalsis through the small bowel, taking up to 50,000 images over an 8-hour period. The patient wears adhesive reception pads that transmit to a small receiver worn on a belt. No food may be consumed for 4 hours; however, liquids can be taken 2 hours after swallowing the capsule. The capsule endoscope is excreted naturally in the feces after a few days. If the capsule is not expelled, an x-ray should be taken to rule out a stricture causing entrapment of the device. The diagnostic capabilities have proven effective even when a negative finding was noted by other means.8 This method of video endoscopy is useful for patients who weigh more than 20 kg and children older than 10 years.19
Endoscopy and robotic-assisted surgery
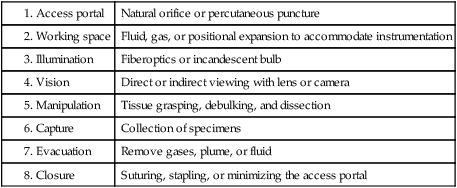
Eight essential elements of endoscopy
Access portal into the body
Working space within the body
Illumination of the working space
Vision within the working space

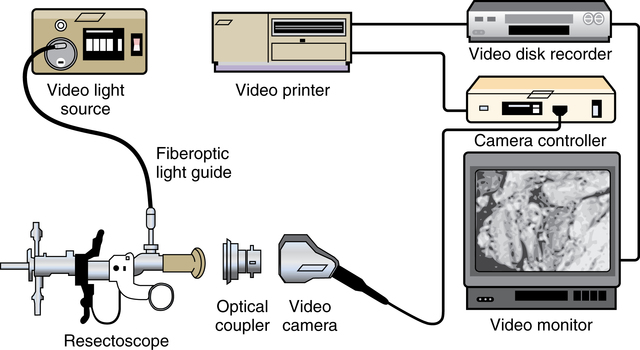
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree



 Website
Website