CHAPTER 22 Endocytosis and the Endosomal Membrane System
Regulated entry of small and large molecules into eukaryotic cells occurs at the plasma membrane, the interface between the intracellular and extracellular environments. Small molecules such as amino acids, sugars, and ions traverse the plasma membrane through the action of integral membrane protein pumps (see Chapter 8), carriers (see Chapter 9), or channels (see Chapter 10), but macromolecules can enter cells only by being captured and enclosed within membrane-bound carriers that invaginate and pinch off the plasma membrane in a process known as endocytosis. Cells use endocytosis to feed themselves, to defend themselves, and to maintain homeostasis. Some toxins, viruses, pathogenic bacteria, and protozoa “hijack” this process to enter cells.
Endocytosis was discovered more than a century ago in white blood cells (macrophages and neutrophils), the body’s “professional phagocytes” (see Fig. 28-8). Endocytosis by these cells is very active, as they internalize the equivalent of their entire plasma membrane surface every hour. It was discovered that when macrophages internalize small particles of blue litmus paper, the color changes, revealing that endocytic vacuoles are acidic. Investigators still use molecules tagged with fluorescent dyes, green fluorescent protein, or electron-dense markers to follow endocytosis in living or fixed cells by light or electron microscopy. Subcellular fractionation, sometimes aided by loading cells with tracers that alter the density of the endocytic compartments or with ferromagnetic tags, has enabled the isolation and biochemical characterization of distinct classes of endocytic structures. In vitro reconstitution systems have also helped to decipher the mechanisms governing membrane trafficking along the endocytic pathway.
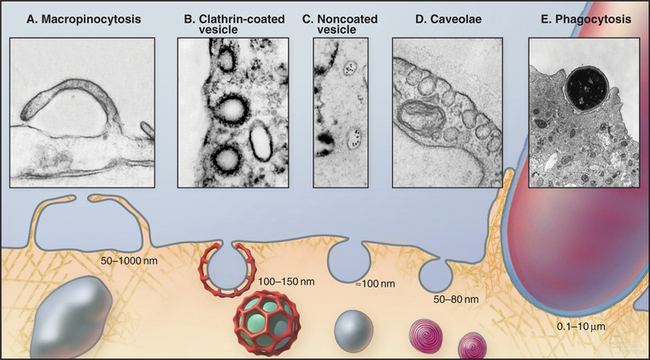
Cells utilize many different mechanisms for endocytosis (Fig. 22-1). These differ in mode of uptake and in the type and intracellular fate of internalized cargo. The mechanisms include phagocytosis, macropinocytosis, clathrin-mediated endocytosis, caveolae-dependent uptake, and nonclathrin/noncaveolae endocytosis. The protrusions or invaginations of the plasma membrane that are formed during these diverse endocytic processes all require coordinated interactions between a variety of protein and lipid molecules that dynamically link the plasma membrane and cortical actin cytoskeleton.
In phagocytosis and clathrin-mediated endocytosis, cell surface receptors selectively bind macromolecules (ligands) to be internalized. Ligands can be proteins, glycoproteins, or carbohydrates. In phagocytosis, the ligands are usually membrane constituents of other cells, bacteria, or viruses. After ligand-receptor complexes are concentrated into patches in the membrane, the membrane is then either pinched off to form small vesicles (in clathrin-mediated endocytosis) or zippered up around the particle to form a large vacuole inside the cell (in phagocytosis).
Phagocytosis
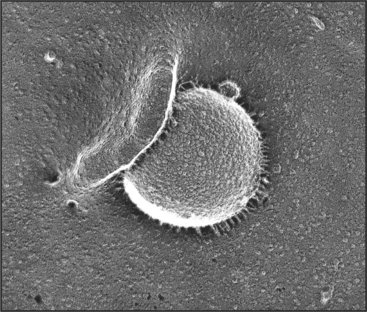
Phagocytosis is the ingestion of large particles such as bacteria, foreign bodies, and remnants of dead cells (Fig. 22-2). Cells use the actin cytoskeleton to push a protrusion of the plasma membrane to surround these particles.
Some cells, including macrophages, dendritic cells, and neutrophils, are specialized for phagocytosis. The presence of bacteria or protozoa in tissues attracts professional phagocytes from the blood (see Fig. 30-13), where they ingest the microorganisms and initiate inflammatory and immune responses. Other cell types use phagocytosis to remove dead neighboring cells, while amoeba use phagocytosis for feeding.
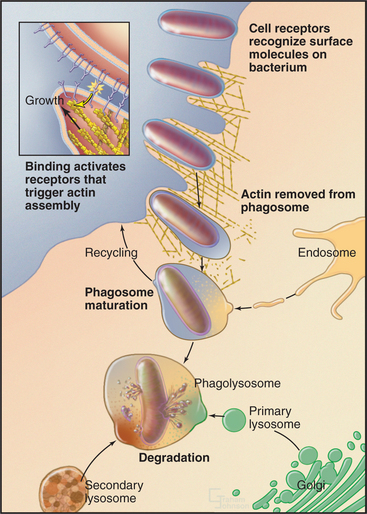
Phagocytosis proceeds through four steps: attachment, engulfment, fusion with lysosomes, and degradation (Fig. 22-3). These steps are highly regulated by cell surface receptors, phospholipids, and signaling cascades mediated by Rho-family GTPases.
Attachment
Attachment depends on the ability of the phagocytic cell to recognize the particle to be ingested. Such specific interactions trigger ingestion of the particle. Vertebrates use proteins, collectively called “opsonins,” to mark bacteria and other foreign particles for phagocytosis. Opsonins include antibodies, which bind to foreign antigens on bacteria, and complement proteins, which tag infected or dying cells. Phagocytes such as macrophages use plasma membrane receptors to bind particles coated with opsonins. For example, immunoglobulin Fc receptors bind to the constant regions of immunoglobulin G molecules (see H3 and H4 domains in Fig. 3-13B) coating pathogenic bacteria and viruses.
Engulfment
Binding of receptors such as the Fc receptor to a foreign particle generates localized signals on the cytoplasmic side of the plasma membrane. These signals trigger the assembly of the actin filaments immediately adjacent to the particle to be ingested. Growth of these actin filaments supports the plasma membrane as it zippers tightly around the particle to form a cup-like protrusion, called the phagocytic cup. The signaling pathways that give rise to these events are dependent on polyphosphatidylinositides and phosphatidylinositol (PI) kinases (Box 22-1 and Fig. 22-4). In the phagocytic cup, PI(3) kinase generates PI(3,4,5)P3 (PIP3). Effectors of this lipid include a group of PH-domain containing GEFs for the small GTPases Rac1, Arf6, and Cdc42. Once these GTPases are activated, they stimulate the cytoskeletal rearrangements of actin, leading to phagocytic cup growth. The requirement of PI(3) kinase is restricted to the stage at which the phagocytic cup seals to form a phagosome. Following this, PIP3 levels in the newly formed phagosome decline rapidly owing to the activity of PI phosphatases. The phosphatase activity leads to more PI(4,5)P2 in the phagosome membrane, which promotes assembly of actin filaments that drive the vesicle away from the plasma membrane.
BOX 22-1 Polyphosphatidylinositides in Endocytosis
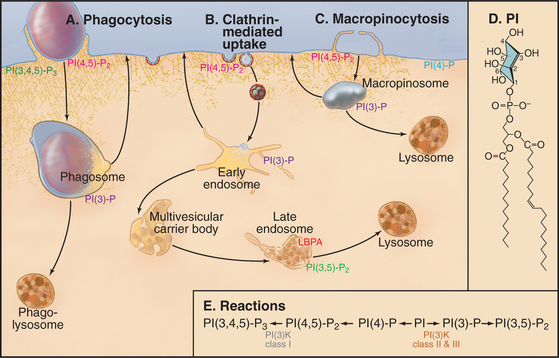
Phosphatidylinositol (PI) is a glycerolphospholipid with a cyclohexanol head group (Fig. 22-4D) that can be phosphorylated on carbons 3, 4, and 5 either singly or in combination to produce polyphosphoinositides (see Fig. 26-7). Polyphosphoinositides are minor lipids in the cytoplasmic leaflet of the plasma membrane (˜1% of total lipids) and endocytic membranes, but lipid kinases and phosphatases can change polyphosphoinositide levels rapidly at local sites in membranes (Fig. 22-4E). This local synthesis of particular polyphosphoinositides regulates membrane remodeling during exocytosis, endocytosis, and vesicular trafficking by recruiting and/or activating proteins that sense the curvature of the lipid bilayer, form scaffolds on the membrane (e.g., clathrin and dynamin), or regulate actin assembly.
The most important polyphosphoinositide for endocytosis is phosphatidylinositol(4,5)bis-phosphate (PI(4,5)P2) with phosphates on carbons 4 and 5 of the head group. Two lipid kinases synthesize PI(4,5)P2 by adding phosphate first to the hydroxyl on carbon 4 and then on carbon 5 (Fig. 22-4E; also see Fig. 26-7). The second enzyme, phosphatidylinositol-4-P-5 kinase, is activated by another glycerolphospholipid, phosphatidic acid (PA; see Fig. 7-2). Since PI(4,5)P2 activates the phospholipase D (see Fig. 26-7) that makes PA, the two enzymes make a positive feedback loop that enriches PI(4,5)P2 locally in the membrane. Interactions of PI(4,5)P2 with proteins from the cytoplasm retard its mobility in the plane of the membrane, raising its local concentration until it is depleted by removal of the head group or by dephosphorylation (see Fig. 26-7).
PI(4,5)P2 participates in clathrin-mediated endocytosis, phagocytosis, and macropinocytosis (Fig. 22-4A-C). The formation of the clathrin lattice and its tethering to the plasma membrane relies on several proteins that interact with PI(4,5)P2, including AP180/CALM, epsin, and AP2 (Fig. 22-9). The GTPase dynamin, which is essential for the scission of clathrin-coated vesicles, also binds to PI(4,5)P2 (Fig. 22-10). Dephosphorylation of PI(4,5)P2 into PI(4)P is mediated by synaptojanin, which plays an important role in clathrin uncoating.
Whereas PI(4,5)P2 helps to regulate endocytosis, PI(3)P is important for early endosome dynamics. It is found on the limiting and intralumenal membranes of endosomes, where it recruits effector molecules. These include EEA1, which is responsible for endosome-endosome fusion through its interaction with Rab5, and Hrs, which recognizes ubiquitinated endocytic cargo and facilitates the formation of intralumenal endosomal vesicles through the assembly of ESCRT-I, -II, and -III. PI(3)kinase Class II or III is responsible for generating PI(3)P on membranes (Fig. 22-4E).
The plasma membrane alone was originally thought to contribute all of the membrane to make a phagocytic cup, but internal membranes are now known to contribute. Internal membranes from recycling endosomes, late endosomes, and possibly ER contribute to the phagocytic cup by fusing with the plasma membrane in a process called focal exocytosis. When secretory lysosomes fuse at the forming phagocytic cup, they release cytokines that contribute to inflammation. This couples phagocytosis to the immune response. Focal exocytosis relies on the same steps that are involved in other membrane fusion events, including transport of internal membranes along cytoskeletal tracks and their fusion by compartment-specific SNAREs under the control of Rab GTPases (see Fig. 21-12).
Fusion with Lysosomes
After closure, the actin filaments surrounding the phagosome disassemble, and motors direct the phagosome along microtubules deep into the cell during a process termed directed maturation. A series of fusion and fission reactions remove plasma membrane components and replace them with endosome-specific components including proteins (e.g., SNAREs) required for selective fusion with acidic lysosomes containing active hydrolytic enzymes. Fusion with lysosomes creates a hybrid vacuole called a phagolysosome (Fig. 22-3).
Alternative Fates of Ingested Particles
Many ingested particles are degraded in phagolysosomes to their constituent amino acids, monosaccharides and disaccharides, nucleotides, and lipids by lysosomal hydrolases. These small products of digestion are transported across the phagolysosomal membrane into the cytoplasm, where they can be reused to synthesize new macromolecules. Any undegraded material re-mains within the lysosome, which is called a residual body.
Antigen-presenting phagocytic cells, such as dendritic cells, cleave proteins of ingested microorganisms into small peptides for loading onto membrane receptors called major histocompatibility complex (MHC) class II molecules. This transfer occurs in phagolysosomes called the antigen-presenting compartment in these cells. MHC Class II molecules loaded with peptides recycle back to the surface of phagocytic cells, where they activate CD4+ T-lymphocytes (see Fig. 27-8).
Some pathogens have counterstrategies to avoid destruction by phagocytes. These include mechanisms to inhibit fusion of phagosomes with lysosomes, to resist the low pH environment of the lysosome, and to escape to the cytoplasm by lysing the phagolysosome membrane (Table 22-1). For example, in tuberculosis, macrophages in the lung phagocytose the bacterium Mycobacterium tuberculosis, but the bacterium evades destruction by secreting a phosphatase that dephosphorylates phosphatidylinositol (3P) and thus halts phagosome maturation.
Table 22-1 SURVIVAL STRATEGIES FOR INTRACELLULAR PATHOGENS
| “Escape” |
| Secretion of toxins that disrupt phagosomal membrane (Shigella flexneri, Listeria monocytogenes, Rickettsia rickettsii) |
| “Dodge” |
| Entrance through alternative, pathogen-specific pathway (Salmonella typhimurium, Legionella pneumophila, Chlamydia trachomatis) |
| Inhibition of phagosome-lysosome fusion (S. typhimurium, Mycobacterium tuberculosis) |
| Inhibition of phagolysosome acidification (Mycobacterium species) |
| “Stand and Fight” |
| Low pH-dependent replication (Coxiella burnetii, S. typhimurium) |
| Enhancement of DNA repair to survive oxidative stress (S. typhimurium) |
| Protective pathogen-specific virulence factors (C. burnetii, S. typhimurium) |
| Prevention of the processing and presentation of bacterial antigens (S. typhimurium) |
Macropinocytosis
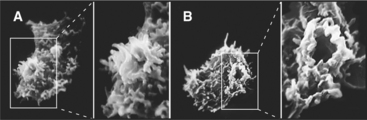
Many cells ingest extracellular fluid in large endocytic structures called macropinosomes. Growth factors or other signals stimulate actin-driven protrusions of the plasma membrane in the form of ruffles (Fig. 22-5). These protrusions close around extracellular fluid, forming a macropinosome, which is then carried along microtubules toward the center of the cell. This allows cells to internalize fluid continuously from their surroundings without concentrating particular molecules, which is useful for bulk nutrient uptake.
Macropinosomes persist inside cells for only about 5 to 20 minutes, during which their membrane components either recycle back to the plasma membrane, potentially bypassing other organelles within the cell, or are delivered to lysosomes (Fig. 22-4). Although the membrane composition of macropinosomes resembles the plasma membrane ruffles from which they were derived, the ruffles themselves are believed to have a different composition from the rest of the plasma membrane by being enriched in both specific polyphosphoinositides and lipid raft markers. Internalization of these membranes during macropinocytosis, therefore, is likely to generate inhomogeneities in the plasma membrane that might influence cellular motility and responses to external stimuli.
Formation of macropinosomes depends on many of the same proteins that are used for phagocytosis. Phosphatidylinositol kinases and GTPases recruit and activate proteins that assemble the actin filaments supporting membrane ruffles. For example, the GTPase Arf6 activates phosphatidyl-4-phosphate-kinase, leading to production of PI(4,5)P2 at plasma membrane sites of macropinocytosis (Fig. 22-4C). PI(4,5)P2 then activates WASp-related proteins and the assembly of actin filaments. Overexpressing a constitutively active form of Arf6 increases ruffling and accumulation of macropinosomes that are enriched in PI(4,5)P2.