Endocrine Glands
PITUITARY GLAND
Structure of the Pituitary Gland
The pituitary gland (formerly called the hypophysis) is a small but mighty structure. It measures only 1.2 to 1.5 cm (about ½ inch) across. By weight, it is even less impressive—only about 0.5 gram (1/60 ounce)! And yet so crucial are the functions of the anterior lobe of the pituitary gland that, in past centuries, it was referred to as the “master gland.”
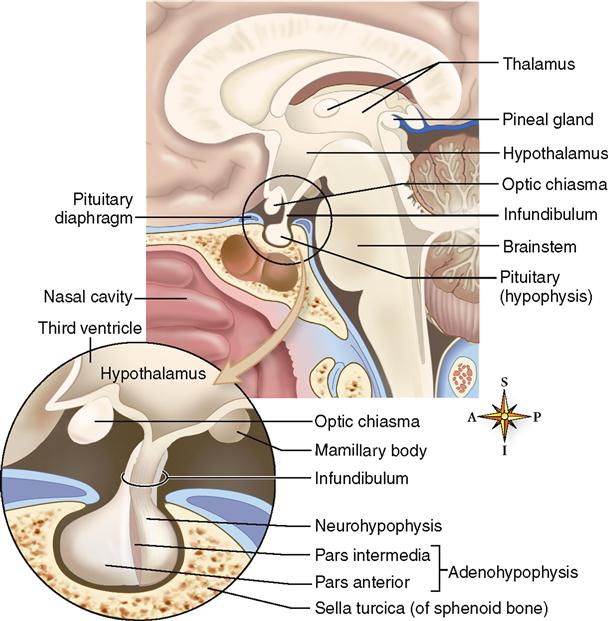
The pituitary gland has a well-protected location within the skull on the ventral surface of the brain (Figure 19-1). It lies in the pituitary fossa of the sella turcica and is covered by a portion of the dura mater called the pituitary diaphragm. The gland has a stemlike stalk, the infundibulum, which connects it to the hypothalamus of the brain.
Although the pituitary looks like one gland, it actually consists of two separate glands—the adenohypophysis, or anterior pituitary gland, and the neurohypophysis, or posterior pituitary gland. In the embryo, the adenohypophysis develops from an upward projection of the pharynx and is composed of regular endocrine tissue. The neurohypophysis, on the other hand, develops from a downward projection of the brain and is composed of neurosecretory tissue. These histological differences are incorporated into their names—adeno means “gland,” and neuro means “nervous.” As you may suspect, the hormones secreted by the adenohypophysis serve very different functions from those released by the neurohypophysis.
Adenohypophysis (Anterior Lobe of Pituitary)
The adenohypophysis, the anterior portion of the pituitary gland, is divided into two parts—the pars anterior and the pars intermedia. The pars anterior forms the major portion of the adenohypophysis and is divided from the tiny pars intermedia by a narrow cleft and some connective tissue (see Figure 19-1).
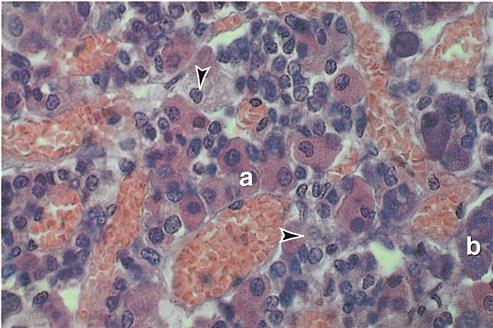
The tissue of the adenohypophysis is composed of irregular clumps of secretory cells supported by fine connective tissue fibers and surrounded by a rich vascular network.
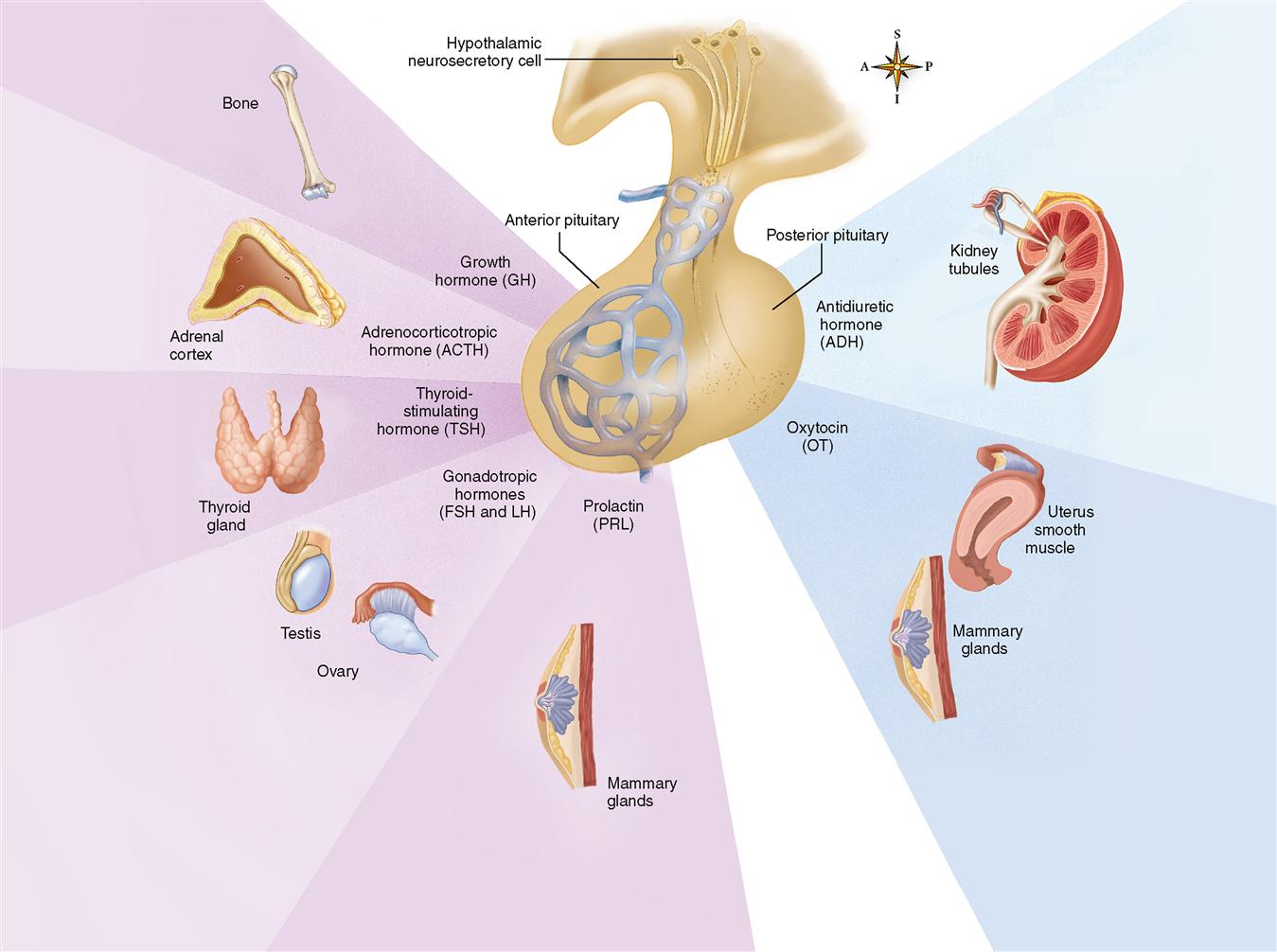
Traditionally, histologists have identified three types of cells according to their affinity for certain types of stains: chromophobes (literally “afraid of color”), acidophils (“acid [stain] lovers”), and basophils (“base [stain] lovers”). All three types are visible in the photomicrograph shown in Figure 19-2. Currently, however, cells of the adenohypophysis are more often classified by their secretions into five types:
1. Somatotrophs—secrete growth hormone (GH)
2. Corticotrophs—secrete adrenocorticotropic hormone (ACTH)
3. Thyrotrophs—secrete thyroid-stimulating hormone (TSH)
4. Lactotrophs—secrete prolactin (PRL)
5. Gonadotrophs—secrete luteinizing hormone (LH) and follicle-stimulating hormone (FSH)
Figure 19-3 summarizes the hormones of the adenohypophysis and shows the primary locations of their target cells.
GROWTH HORMONE
Growth hormone (GH), or somatotropin (STH), promotes bodily growth indirectly by stimulating the liver and other tissues to produce another hormone called insulin-like growth factor 1 (IGF-1), which, in turn, produces most of the effects attributed to GH.
One of the functions of the GH, by way of IGF-1, is to accelerate amino acid transport into cells. Rapid entrance of amino acids from the blood into the cells allows protein anabolism within the cells to accelerate. Increased protein anabolism allows an increased rate of growth. GH promotes the growth of bone, muscle, and other tissues (Box 19-1).
In addition to stimulating protein anabolism, GH also stimulates fat metabolism. GH accelerates mobilization of lipids from storage in adipose cells and also speeds up the catabolism of those lipids after they have entered another cell. In this way, GH tends to shift a cell’s use of nutrients away from carbohydrate (glucose) catabolism and toward lipid catabolism as an energy source. Because less glucose is then removed from the blood by cells, the blood glucose levels tend to rise. Thus GH is said to have a hyperglycemic effect. Insulin (from the pancreas) has the opposite effect—it promotes glucose entry into cells, producing a hypoglycemic effect. Therefore GH and insulin function as antagonists. The balance between these two hormones is vital to maintaining a homeostasis of blood glucose levels.
GH affects metabolism in these ways:
 Promotes protein anabolism (growth, tissue repair)
Promotes protein anabolism (growth, tissue repair)
 Promotes lipid mobilization and catabolism
Promotes lipid mobilization and catabolism
 Indirectly inhibits glucose metabolism
Indirectly inhibits glucose metabolism
PROLACTIN
Prolactin (PRL), produced by acidophils in the pars anterior, is also called lactogenic hormone. Both names of this hormone suggest its function in “generating” or initiating milk secretion (lactation). During pregnancy, a high level of PRL promotes the development of the breasts in anticipation of milk secretion. At the birth of an infant, PRL in the mother stimulates the mammary glands to begin milk secretion.
Hypersecretion of PRL may cause lactation in nonnursing women, disruption of the menstrual cycle, and impotence in men. Hyposecretion of PRL is usually insignificant except in women who want to nurse their children. Milk production cannot be initiated or maintained without PRL.
TROPIC HORMONES
Tropic hormones are hormones that have a stimulating effect on other endocrine glands. These hormones stimulate the development of their target glands and tend to stimulate synthesis and secretion of the target hormone (Box 19-2). Four principal tropic hormones are produced and secreted by the basophils of the pars anterior:
FSH and LH are called gonadotropins because they stimulate the growth and maintenance of the gonads (ovaries and testes). During childhood the adenohypophysis secretes insignificant amounts of the gonadotropins. A few years before puberty, gonadotropin secretion is gradually increased. Then, suddenly, their secretion spurts, and the gonads are stimulated to develop and begin their normal functions.
In addition to those listed, the adenohypophysis produces many other hormones in small amounts. Many of these are also produced elsewhere in the body. For example, α-MSH (alpha melanocyte-stimulating hormone) and other melanocortins discussed in Chapter 7 (see p. 179) are produced in the skin and other tissues, with a relatively insignificant amount also produced in the adenohypophysis.
CONTROL OF SECRETION IN THE ADENOHYPOPHYSIS
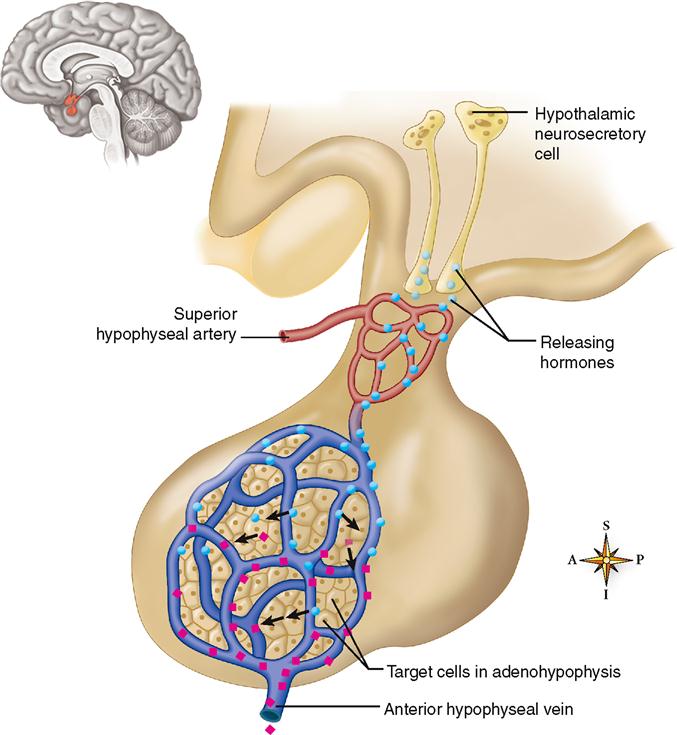
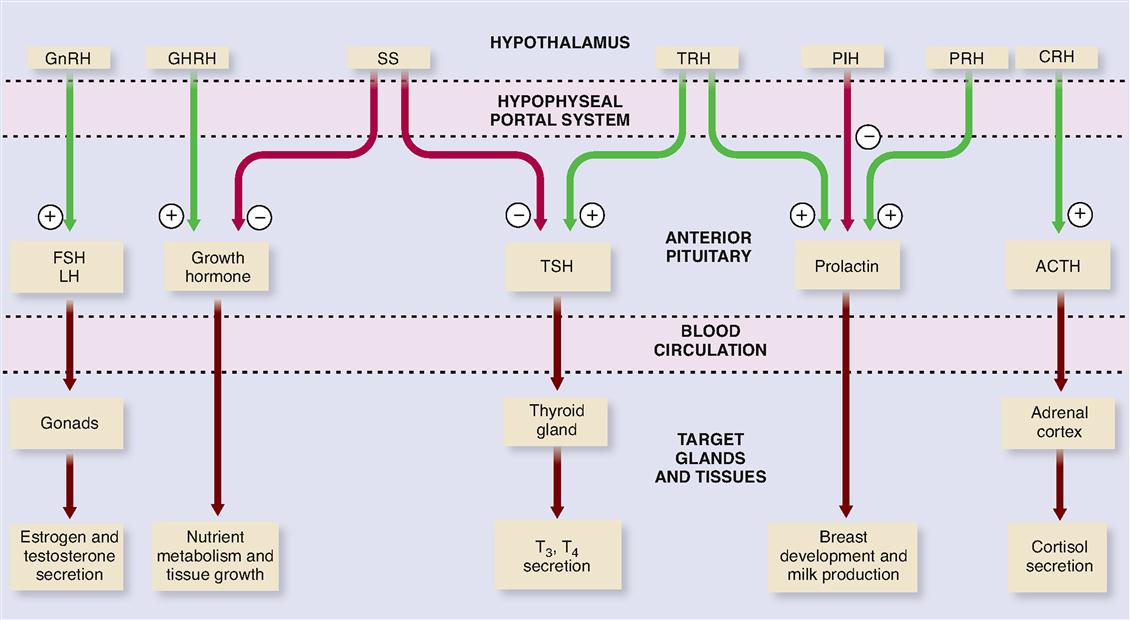
The cell bodies of neurons in certain parts of the hypothalamus synthesize chemicals that their axons then secrete into the blood. These chemicals, generally called releasing hormones, travel through a complex of small blood vessels called the hypophyseal portal system (Figure 19-4). A portal system is an arrangement of blood vessels in which blood exiting one tissue is immediately carried to a second tissue before being returned to the heart and lungs for oxygenation and redistribution. The hypophyseal portal system carries blood from the hypothalamus directly to the adenohypophysis, where the target cells of the releasing hormones are located. The advantage of a portal system in the hypophysis is that a small amount of hormone can be delivered directly to its target tissue without the great dilution that would occur in the general circulation. The releasing hormones that arrive in the adenohypophysis by means of this portal system influence the secretion of hormones by acidophils and basophils. In this manner, the hypothalamus directly regulates the secretion of the adenohypophysis. You can see that the supposed “master gland” really has a master of its own—the hypothalamus.
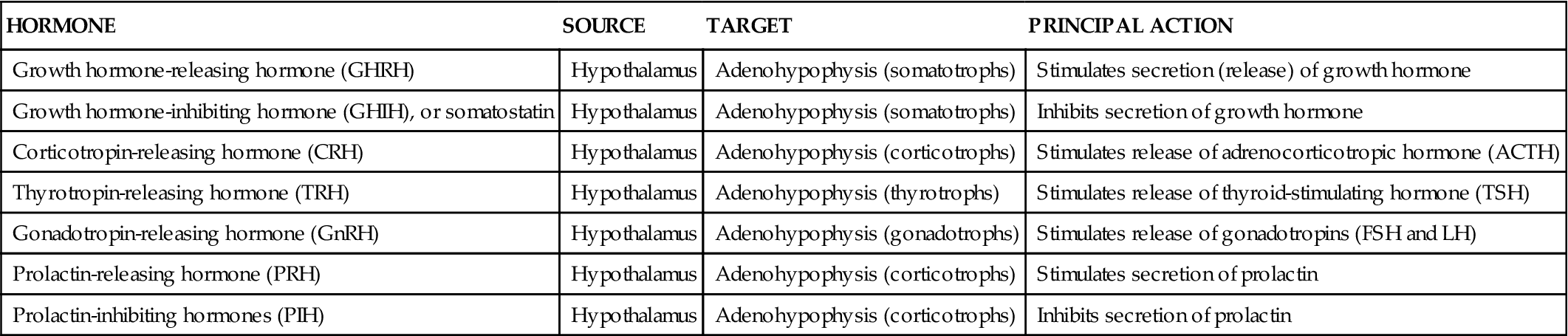
The following is a list of some of the important hormones secreted by the hypothalamus into the hypophyseal portal system:
 Growth hormone–releasing hormone (GHRH)
Growth hormone–releasing hormone (GHRH)
 Growth hormone–inhibiting hormone (GHIH) (also called somatostatin [SS])
Growth hormone–inhibiting hormone (GHIH) (also called somatostatin [SS])
 Corticotropin-releasing hormone (CRH)
Corticotropin-releasing hormone (CRH)
 Thyrotropin-releasing hormone (TRH)
Thyrotropin-releasing hormone (TRH)
 Gonadotropin-releasing hormone (GnRH)
Gonadotropin-releasing hormone (GnRH)
 Prolactin-releasing hormone (PRH)
Prolactin-releasing hormone (PRH)
Figure 19-5 and Table 19-1 list functions of each releasing hormone. Before consulting the figure or table, try to deduce their functions from their names.
TABLE 19-1
| HORMONE | SOURCE | TARGET | PRINCIPAL ACTION |
| Growth hormone-releasing hormone (GHRH) | Hypothalamus | Adenohypophysis (somatotrophs) | Stimulates secretion (release) of growth hormone |
| Growth hormone-inhibiting hormone (GHIH), or somatostatin | Hypothalamus | Adenohypophysis (somatotrophs) | Inhibits secretion of growth hormone |
| Corticotropin-releasing hormone (CRH) | Hypothalamus | Adenohypophysis (corticotrophs) | Stimulates release of adrenocorticotropic hormone (ACTH) |
| Thyrotropin-releasing hormone (TRH) | Hypothalamus | Adenohypophysis (thyrotrophs) | Stimulates release of thyroid-stimulating hormone (TSH) |
| Gonadotropin-releasing hormone (GnRH) | Hypothalamus | Adenohypophysis (gonadotrophs) | Stimulates release of gonadotropins (FSH and LH) |
| Prolactin-releasing hormone (PRH) | Hypothalamus | Adenohypophysis (corticotrophs) | Stimulates secretion of prolactin |
| Prolactin-inhibiting hormones (PIH) | Hypothalamus | Adenohypophysis (corticotrophs) | Inhibits secretion of prolactin |
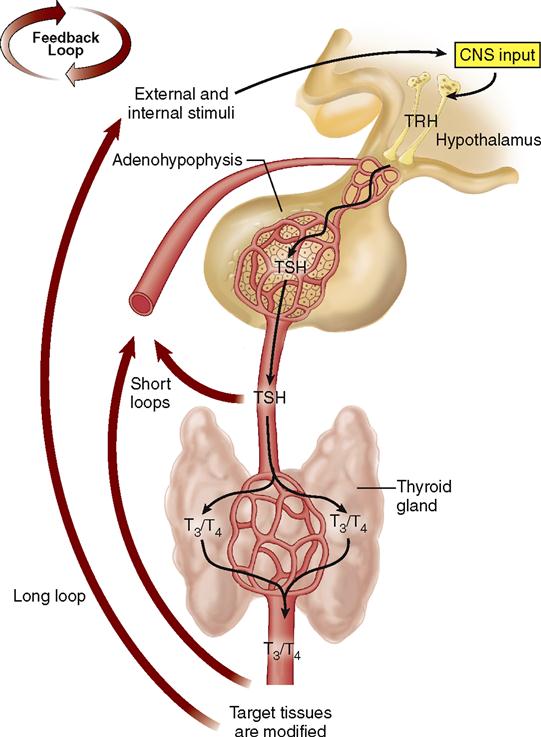
Through negative feedback mechanisms, the hypothalamus adjusts the secretions of the adenohypophysis, and the adenohypophysis adjusts the secretions of its target glands, which in turn adjust the activity of their target tissues (Box 19-3). For example, Figure 19-6 shows the negative feedback control of the secretion of TSH and thyroid hormone (T3 and T4).
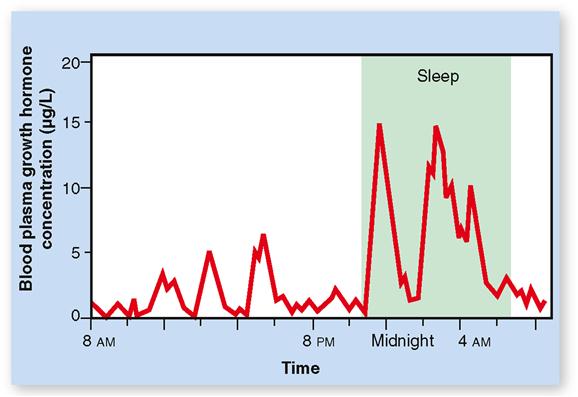
Hormone secretion can occur in pulses or peaks, as we see in a graph of minute by minute variations in GH secretion (Figure 19-7). The peaks result from many somatotroph cells in the pituitary collectively increasing their rate of secretion of GH. Such increases result from pulses in GHRH secretion by the hypothalamus (see Figure 19-5), which are especially large during sleep. Exercise, stress, and high-protein meals can cause an increase in the frequency of these peaks.