CHAPTER 32 Elements of Renal Function
OVERVIEW OF RENAL FUNCTION
Finally, the kidneys are important endocrine organs that produce and secrete renin, calcitriol, and erythropoietin. Renin activates the renin-angiotensin-aldosterone system, which helps regulate blood pressure and Na+ and K+ balance. Calcitriol, a metabolite of vitamin D3, is necessary for the normal absorption of Ca++ by the gastrointestinal tract and for its deposition in bone (see also Chapter 35). In patients with renal disease, the kidneys’ ability to produce calcitriol is impaired, and levels of this hormone are reduced. As a result, Ca++ absorption by the intestine is decreased. This reduced intestinal Ca++ absorption contributes to the bone formation abnormalities seen in patients with chronic renal disease. Another consequence of many kidney diseases is a reduction in erythropoietin production and secretion. Erythropoietin stimulates red blood cell formation by the bone marrow. Decreased erythrocyte production contributes to the anemia that occurs in chronic renal failure.
Kidney disease is a major health problem. In the United States:
FUNCTIONAL ANATOMY OF THE KIDNEYS
Gross Anatomy
The gross anatomic features of the human kidney are illustrated in Figure 32-1. The medial side of each kidney contains an indentation through which pass the renal artery and vein, nerves, and pelvis. If a kidney were cut in half, two regions would be evident: an outer region called the cortex and an inner region called the medulla. The cortex and medulla are composed of nephrons (the functional units of the kidney), blood vessels, lymphatics, and nerves. The medulla in the human kidney is divided into conical masses called renal pyramids. The base of each pyramid originates at the corticomedullary border, and the apex terminates in a papilla, which lies within a minor calyx. Minor calyces collect urine from each papilla. The numerous minor calyces expand into two or three open-ended pouches, the major calyces. The major calyces in turn feed into the pelvis. The pelvis represents the upper, expanded region of the ureter, which carries urine from the pelvis to the urinary bladder. The walls of the calyces, pelvis, and ureters contain smooth muscle that contracts to propel the urine toward the urinary bladder.
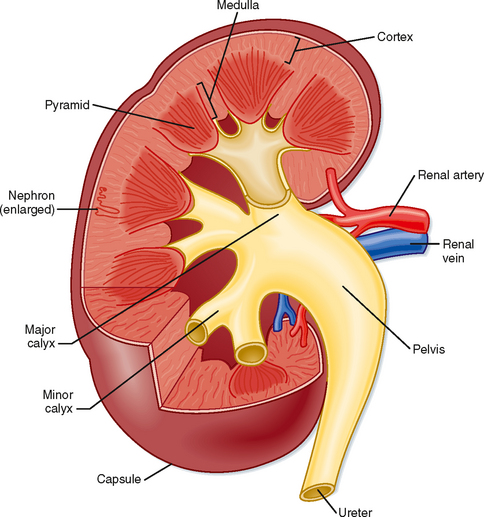
Figure 32-1 Structure of a human kidney, cut open to show the internal structures.
(Modified from Marsh DJ: Renal Physiology. New York, Raven, 1983.)
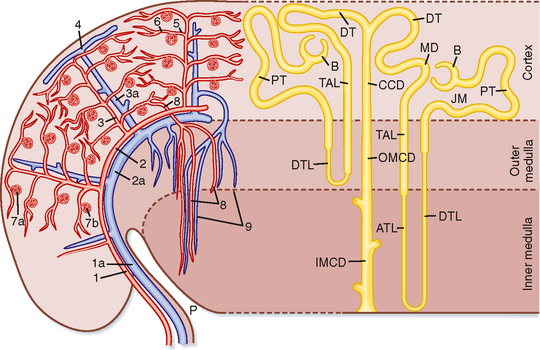
Blood flow to the two kidneys is equivalent to about 25% (1.25 L/min) of the cardiac output in resting individuals. However, the kidneys constitute less than 0.5% of total body weight. As illustrated in Figure 32-2 (left), the renal artery branches progressively to form the interlobar artery, the arcuate artery, the interlobular artery, and the afferent arteriole, which leads into the glomerular capillaries (i.e., glomerulus). The glomerular capillaries come together to form the efferent arteriole, which leads into a second capillary network, the peritubular capillaries, which supply blood to the nephron. The vessels of the venous system run parallel to the arterial vessels and progressively form the interlobular vein, arcuate vein, interlobar vein, and renal vein, which courses beside the ureter.
Ultrastructure of the Nephron
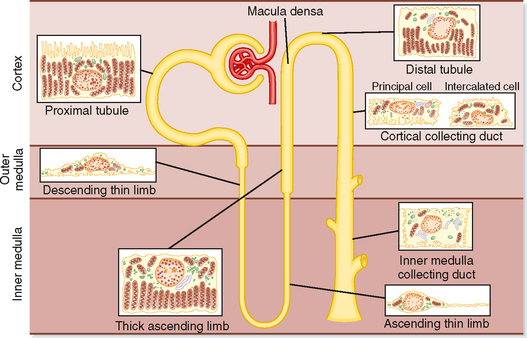
The functional unit of the kidneys is the nephron. Each human kidney contains approximately 1.2 million nephrons, which are hollow tubes composed of a single cell layer. The nephron consists of a renal corpuscle, proximal tubule, loop of Henle, distal tubule, and collecting duct system* (Fig. 32-3; also see Fig. 32-4). The renal corpuscle consists of glomerular capillaries and Bowman’s capsule. The proximal tubule initially forms several coils, followed by a straight piece that descends toward the medulla. The next segment is the loop of Henle, which is composed of the straight part of the proximal tubule, the descending thin limb (which ends in a hairpin turn), the ascending thin limb (only in nephrons with long loops of Henle), and the thick ascending limb. Near the end of the thick ascending limb, the nephron passes between the afferent and efferent arterioles of the same nephron. This short segment of the thick ascending limb is called the macula densa. The distal tubule begins a short distance beyond the macula densa and extends to the point in the cortex where two or more nephrons join to form a cortical collecting duct. The cortical collecting duct enters the medulla and becomes the outer medullary collecting duct and then the inner medullary collecting duct.
Each nephron segment is made up of cells that are uniquely suited to perform specific transport functions (Fig. 32-3). Proximal tubule cells have an extensively amplified apical membrane (the urine side of the cell) called the brush border, which is present only in the proximal tubule. The basolateral membrane (the blood side of the cell) is highly invaginated. These invaginations contain many mitochondria. In contrast, the descending and ascending thin limbs of Henle’s loop have poorly developed apical and basolateral surfaces and few mitochondria. The cells of the thick ascending limb and the distal tubule have abundant mitochondria and extensive infoldings of the basolateral membrane.
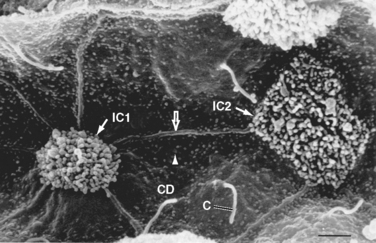
The collecting duct is composed of two cell types: principal cells and intercalated cells. Principal cells have a moderately invaginated basolateral membrane and contain few mitochondria. Principal cells play an important role in reabsorption of NaCl (see Chapter 33 and 34) and secretion of K+ (see Chapter 35). Intercalated cells, which play an important role in regulating acid-base balance, have a high density of mitochondria. One population of intercalated cells secretes H+ (i.e., reabsorbs HCO3−), and a second population secretes HCO3− (see Chapter 36). The final segment of the nephron, the inner medullary collecting duct, is composed of inner medullary collecting duct cells. Cells of the inner medullary collecting duct have poorly developed apical and basolateral surfaces and few mitochondria.
All cells in the nephron, except intercalated cells, have in their apical plasma membrane a single nonmotile primary cilium that protrudes into the tubule fluid (Fig. 32-4). Primary cilia are mechanosensors (i.e., they sense changes in the rate of flow of tubule fluid) and chemosensors (i.e., they sense or respond to compounds in the surrounding fluid), and they initiate Ca++-dependent signaling pathways, including those that control kidney cell function, proliferation, differentiation, and apoptosis (i.e., programmed cell death).
Polycystin 1 (encoded by the PKD1 gene) and polycystin 2 (encoded by the PKD2 gene) are expressed in the membrane of primary cilia and mediate entry of Ca++ into cells. PKD1 and PKD2 are thought to play an important role in flow-dependent K+ secretion by principal cells of the collecting duct. As described in more detail in Chapter 35, increased flow of tubule fluid in the collecting duct is a strong stimulus for secretion of K+. Increased flow bends the primary cilium in principal cells, which activates the PKD1/PKD2 Ca++ conducting channel complex and allows Ca++ to enter the cell and increase intracellular [Ca++]. The increase in [Ca++] activates K+ channels in the apical plasma membrane, which enhances secretion of K+ from the cell into the tubule fluid.
Nephrons may be subdivided into superficial and juxtamedullary types (Fig. 32-2). The renal corpuscle of each superficial nephron is located in the outer region of the cortex. Its loop of Henle is short, and its efferent arteriole branches into peritubular capillaries that surround the nephron segments of its own and adjacent nephrons. This capillary network conveys oxygen and important nutrients to the nephron segments in the cortex, delivers substances to the nephron for secretion (i.e., movement of a substance from blood into tubular fluid), and serves as a pathway for return of reabsorbed water and solutes to the circulatory system. A few species, including humans, also possess very short superficial nephrons whose loops of Henle never enter the medulla.
The renal corpuscle of each juxtamedullary nephron is located in the region of the cortex adjacent to the medulla (Fig. 32-2, right). When compared with superficial nephrons, juxtamedullary nephrons differ anatomically in two important ways: the loop of Henle is longer and extends deeper into the medulla, and the efferent arteriole forms not only a network of peritubular capillaries but also a series of vascular loops called the vasa recta.
As shown in Figure 32-2, the vasa recta descend into the medulla, where they form capillary networks that surround the collecting ducts and ascending limbs of the loop of Henle. The blood returns to the cortex in the ascending vasa recta. Although less than 0.7% of the renal blood flow (RBF) enters the vasa recta, these vessels subserve important functions in the renal medulla, including (1) conveying oxygen and important nutrients to nephron segments, (2) delivering substances to the nephron for secretion, (3) serving as a pathway for the return of reabsorbed water and solutes to the circulatory system, and (4) concentrating and diluting the urine (urine concentration and dilution are discussed in more detail in Chapter 34).
Ultrastructure of the Renal Corpuscle
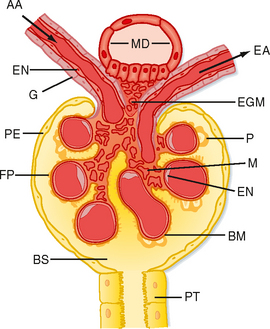
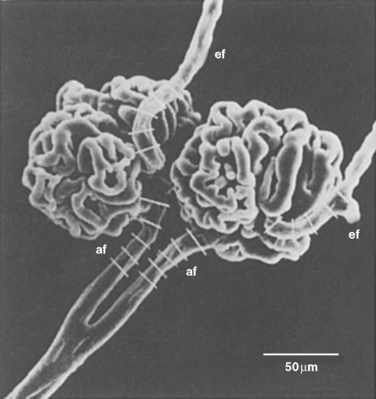
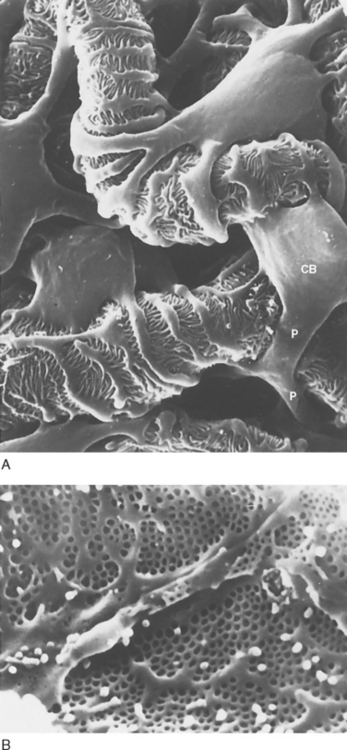
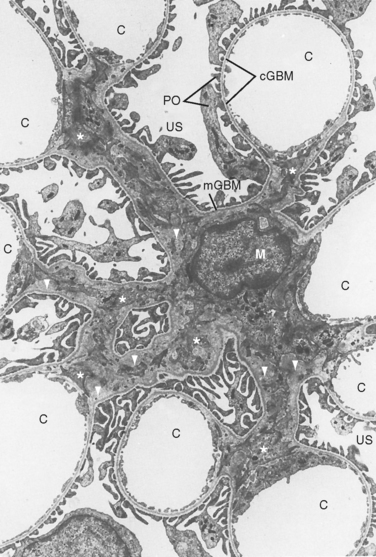
The first step in urine formation begins with passive movement of a plasma ultrafiltrate from the glomerular capillaries (i.e., glomerulus) into Bowman’s space. The term ultrafiltration refers to the passive movement of an essentially protein-free fluid from the glomerular capillaries into Bowman’s space. To appreciate the process of ultrafiltration one must understand the anatomy of the renal corpuscle. The glomerulus consists of a network of capillaries supplied by the afferent arteriole and drained by the efferent arteriole (Figs. 32-5 and 32-6). During embryological development, the glomerular capillaries press into the closed end of the proximal tubule to form the Bowman capsule of a renal corpuscle. The capillaries are covered by epithelial cells called podocytes that form the visceral layer of Bowman’s capsule (Figs. 32-7 through 32-9). The visceral cells face outward at the vascular pole (i.e., where the afferent and efferent arterioles enter and exit Bowman’s capsule) to form the parietal layer of Bowman’s capsule. The space between the visceral layer and the parietal layer is Bowman’s space, which at the urinary pole (i.e., where the proximal tubule joins Bowman’s capsule) of the glomerulus becomes the lumen of the proximal tubule.
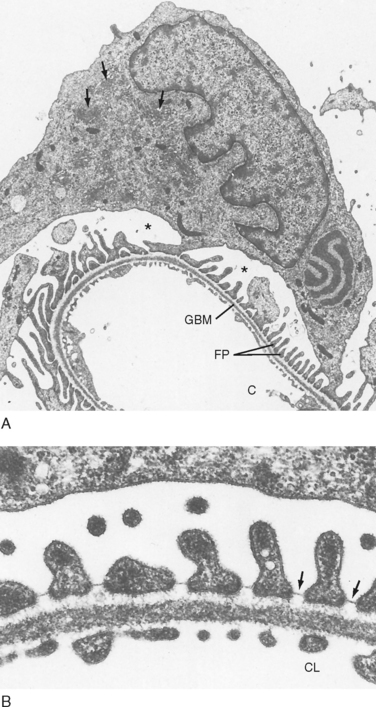
The endothelial cells of glomerular capillaries are covered by a basement membrane that is surrounded by podocytes (Figs. 32-5 and 32-7 to 32-9). The capillary endothelium, basement membrane, and foot processes of podocytes form the so-called filtration barrier (Figs. 32-5 and 32-7 to 32-9). The endothelium is fenestrated (i.e., contains 700-Å holes, where 1 Å = 10−10 m) and freely permeable to water, small solutes (such as Na+, urea, and glucose), and most proteins but is not permeable to red blood cells, white blood cells, or platelets. Because endothelial cells express negatively charged glycoproteins on their surface, they may retard the filtration of very large anionic proteins into Bowman’s space. In addition to their role as a barrier to filtration, the endothelial cells synthesize a number of vasoactive substances (e.g., nitric oxide [NO], a vasodilator, and endothelin-1 [ET-1], a vasoconstrictor) that are important in controlling renal plasma flow (RPF).
The basement membrane, which is a porous matrix of negatively charged proteins, including type IV collagen, laminin, the proteoglycans agrin and perlecan, and fibronectin, is an important filtration barrier to plasma proteins. The basement membrane is thought to function primarily as a charge-selective filter in which the ability of proteins to cross the filter is based on charge.*
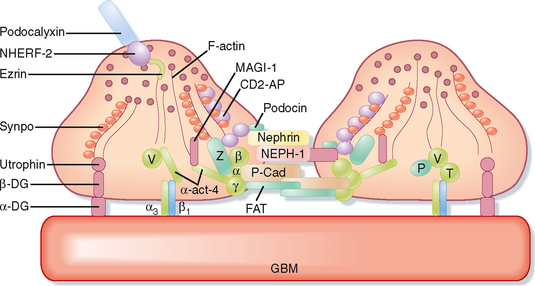
The podocytes, which are endocytic, have long finger-like processes that completely encircle the outer surface of the capillaries (Fig. 32-8). The processes of the podocytes interdigitate to cover the basement membrane and are separated by apparent gaps called filtration slits. Each filtration slit is bridged by a thin diaphragm that contains pores with a dimension of 40 × 140 Å. The filtration slit diaphragm, which appears as a continuous structure when viewed by electron microscopy (Fig. 32-7, B), is composed of several proteins, including nephrin (NPHS1), NEPH-1, podocin (NPHS2), α-actinin 4 (ACTN4), and CD2-AP (Figs. 32-10 and 32-11). Filtration slits, which function primarily as a size-selective filter, keep the proteins and macromolecules that cross the basement membrane from entering Bowman’s space.
< div class='tao-gold-member'>
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree