OBJECTIVES
After studying this chapter, you should be able to:
Describe the primary types of rhythms that make up the electroencephalogram (EEG).
List the main clinical uses of the EEG.
Summarize the behavioral and EEG characteristics of each of the stages of nonrapid eye movement (NREM) and rapid eye movement (REM) sleep and the mechanisms responsible for their production.
Describe the pattern of normal nighttime sleep in adults and the variations in this pattern from birth to old age.
Describe the interplay between brainstem neurons that contain norepinephrine, serotonin, and acetylcholine as well as GABA and histamine in mediating transitions between sleep and wakefulness.
Discuss the circadian rhythm and the role of the suprachiasmatic nuclei (SCN) in its regulation.
Describe the diurnal regulation of synthesis of melatonin from serotonin in the pineal gland and its secretion into the bloodstream.
INTRODUCTION
Most of the various sensory pathways described in Chapters 8, 9, 10, 11 relay impulses from sense organs via three- and four-neuron chains to particular sites in the cerebral cortex. The impulses are responsible for perception and localization of individual sensations. However, they must be processed in the awake brain to be perceived. There is a spectrum of behavioral states ranging from deep sleep through light sleep, REM sleep, and the two awake states: relaxed awareness and awareness with concentrated attention. Discrete patterns of brain electrical activity correlate with each of these states. Feedback oscillations within the cerebral cortex and between the thalamus and the cortex serve as producers of this activity and possible determinants of the behavioral state. Arousal can be produced by sensory stimulation and by impulses ascending in the reticular core of the midbrain. Many of these activities have rhythmic fluctuations that are approximately 24 h in length; that is, they are circadian.
THALAMUS, CEREBRAL CORTEX, & RETICULAR FORMATION
The thalamus is a large collection of neuronal groups within the diencephalon; it participates in sensory, motor, and limbic functions. Virtually all information that reaches the cortex is processed by the thalamus, leading to its being called the “gateway to the cerebral cortex.”
The thalamus can be divided into nuclei that project diffusely to wide regions of the neocortex and nuclei that project to specific discrete portions of the neocortex and limbic system. The nuclei that project to wide regions of the neocortex are the midline and intralaminar nuclei. The nuclei that project to specific areas include the specific sensory relay nuclei and the nuclei concerned with efferent control mechanisms. The specific sensory relay nuclei include the medial and lateral geniculate bodies, which relay auditory and visual impulses to the auditory and visual cortices, and the ventral posterior lateral (VPL) and ventral posteromedial nuclei, which relay somatosensory information to the postcentral gyrus. The ventral anterior and ventral lateral nuclei are concerned with motor function. They receive input from the basal ganglia and the cerebellum and project to the motor cortex. The anterior nuclei receive afferents from the mammillary bodies and project to the limbic cortex, which may be involved in memory and emotion. Most of the thalamic nuclei described are excitatory neurons that release glutamate. The thalamus also contains inhibitory neurons in the thalamic reticular nucleus. These neurons release GABA, and unlike the other thalamic neurons just described, their axons do not project to the cortex. Rather, they are thalamic interneurons and modulate the responses of other thalamic neurons to input coming from the cortex.
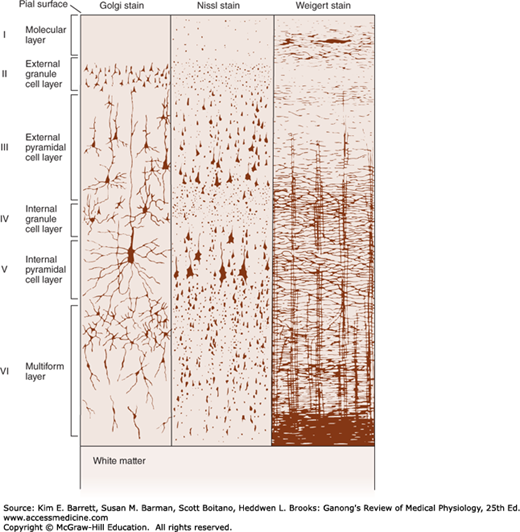
The neocortex is generally arranged in six layers (Figure 14–1). The most common cell type is the pyramidal neuron with an extensive vertical dendritic tree (Figure 14–1 and Figure 14–2) that may reach to the cortical surface. Their cell bodies can be found in all cortical layers except layer I. The axons of these cells usually give off recurrent collaterals that turn back and synapse on the superficial portions of the dendritic trees. Afferents from the specific nuclei of the thalamus terminate primarily in cortical layer IV, whereas the nonspecific afferents are distributed to layers I–IV. Pyramidal neurons are the only projection neurons of the cortex, and they are excitatory neurons that release glutamate at their terminals. The other cortical cell types are local circuit neurons (interneurons) that have been classified based on their shape, pattern of projection, and neurotransmitter. Inhibitory interneurons (basket cells and chandelier cells) release GABA as their neurotransmitter. Basket cells have long axonal endings that surround the soma of pyramidal neurons; they account for most inhibitory synapses on the pyramidal soma and dendrites. Chandelier cells are a powerful source of inhibition of pyramidal neurons because they have axonal endings that terminate exclusively on the initial segment of the pyramidal cell axon. Their terminal boutons form short vertical rows that resemble candlesticks, thus accounting for their name. Spiny stellate cells are excitatory interneurons that release glutamate as a neurotransmitter. These cells are located primarily in layer IV and are a major recipient of sensory information arising from the thalamus; they are an example of a multipolar neuron (Chapter 4) with local dendritic and axonal arborizations.
FIGURE 14–1
Structure of the cerebral cortex. The cortical layers are indicated by the numbers. Golgi stain shows neuronal cell bodies and dendrites, Nissl stain shows cell bodies, and Weigert myelin sheath stain shows myelinated nerve fibers. (Modified with permission from Ranson SW, Clark SL: The Anatomy of the Nervous System, 10th ed. St. Louis, MO: Saunders; 1959.)
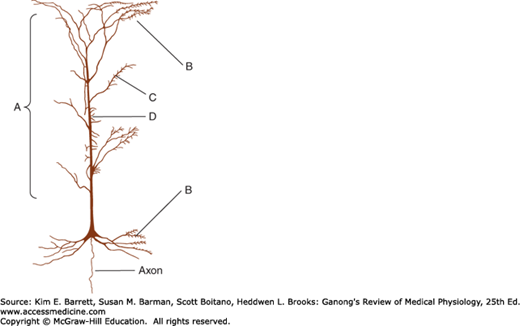
FIGURE 14–2
Neocortical pyramidal cell, showing the distribution of neurons that terminate on it. A denotes nonspecific afferents from the reticular formation and the thalamus; B denotes recurrent collaterals of pyramidal cell axons; C denotes commissural fibers from mirror image sites in the contralateral hemisphere; D denotes specific afferents from thalamic sensory relay nuclei. (Based on Scheibel ME, Scheibel AB: Structural organization of nonspecific thalamic nuclei and their projection toward cortex. Brain Res 1967 Sep; 6(1):60–94.)
In addition to being organized into layers, the cerebral cortex is also organized into columns. Neurons within a column have similar response properties, suggesting they comprise a local processing network (eg, orientation and ocular dominance columns in the visual cortex).
The reticular formation, the phylogenetically old reticular core of the brain, occupies the central portion of the medulla and midbrain, surrounding the fourth ventricle and cerebral aqueduct. The reticular formation contains the cell bodies and fibers of many of the serotonergic, noradrenergic, and cholinergic systems. These pathways were shown in Figure 7–2. The reticular formation also contains many of the areas concerned with regulation of heart rate, blood pressure, and respiration. The reticular formation plays an important role in determining the level of arousal, thus it is called the ascending reticular activating system (RAS).
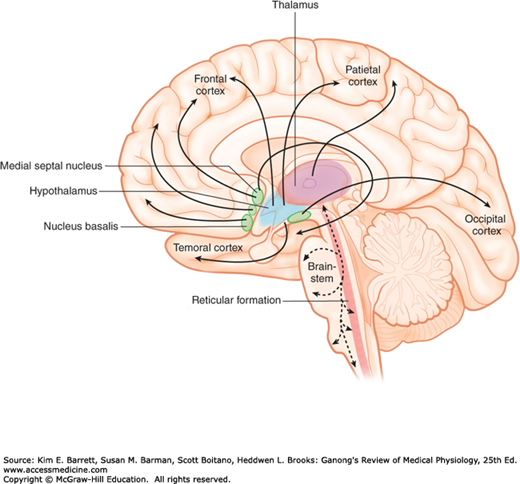
The RAS is a complex polysynaptic pathway arising from the brainstem reticular formation and hypothalamus with projections to the intralaminar and reticular nuclei of the thalamus which, in turn, project diffusely and nonspecifically to wide regions of the cortex including the frontal, parietal, temporal, and occipital cortices (Figure 14–3). Collaterals funnel into it not only from the long ascending sensory tracts but also from the trigeminal, auditory, visual, and olfactory systems. The complexity of the neuron net and the degree of convergence in it abolish modality specificity, and most reticular neurons are activated with equal facility by different sensory stimuli. The system is therefore nonspecific, whereas the classic sensory pathways are specific in that the fibers in them are activated by only one type of sensory stimulation.
FIGURE 14–3
Cross section through the midline of the human brain showing the ascending reticular activating system in the brainstem with projections to the intralaminar nuclei of the thalamus and the output from the intralaminar nuclei to many parts of the cerebral cortex. Activation of these areas can be shown by positive emission tomography scans when subjects shift from a relaxed awake state to an attention-demanding task.
EVOKED CORTICAL POTENTIALS
The electrical events that occur in the cortex after stimulation of a sense organ can be monitored with a recording electrode. If the electrode is over the primary receiving area for a particular sense, a surface-positive wave appears with a latency of 5–12 ms. This is followed by a small negative wave, and then a larger, more prolonged positive deflection frequently occurs with a latency of 20–80 ms. The first positive–negative wave sequence is the primary evoked potential; the second is the diffuse secondary response.
The primary evoked potential is highly specific in its location and can be observed only where the pathways from a particular sense organ end. The positive–negative wave sequence recorded from the surface of the cortex occurs because the superficial cortical layers are positive relative to the initial negativity, then negative relative to the deep hyperpolarization. The surface-positive diffuse secondary response, unlike the primary response, is not highly localized. It appears at the same time over most of the cortex and is due to activity in projections from the midline and related thalamic nuclei.
PHYSIOLOGIC BASIS OF THE ELECTROENCEPHALOGRAM
The background electrical activity of the brain in unanesthetized animals was first described in the 19th century. Subsequently, it was analyzed in a systematic manner by the German psychiatrist Hans Berger, who introduced the term electroencephalogram (EEG) to denote the recording of the variations in brain potential. The EEG can be recorded with scalp electrodes through the unopened skull or with electrodes on or in the brain. The term electrocorticogram is used for the recording obtained with electrodes on the pial surface of the cortex.
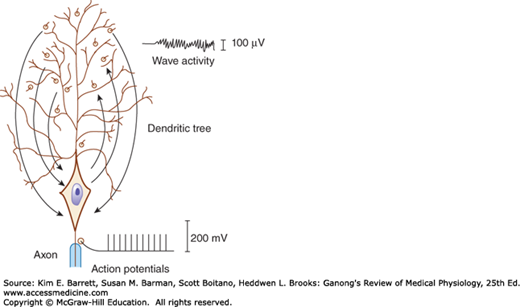
The EEG recorded from the scalp is a measure of the summation of dendritic postsynaptic potentials rather than action potentials (Figure 14–4). The dendrites of the cortical neurons are a forest of similarly oriented, densely packed units in the superficial layers of the cerebral cortex (Figure 14–1). Propagated potentials can be generated in dendrites. In addition, recurrent axon collaterals end on dendrites in the superficial layers. As excitatory and inhibitory endings on the dendrites of each cell become active, current flows into and out of these current sinks and sources from the rest of the dendritic processes and the cell body. The cell body–dendrite relationship is therefore that of a constantly shifting dipole. Current flow in this dipole produces wavelike potential fluctuations in a volume conductor (Figure 14–4). When the sum of the dendritic activity is negative relative to the cell body, the neuron is depolarized and hyperexcitable; when it is positive, the neuron is hyperpolarized and less excitable.
FIGURE 14–4
Diagrammatic comparison of the electrical responses of the axon and the dendrites of a large cortical neuron. Current flow to and from active synaptic knobs on the dendrites produces wave activity, while all-or-none action potentials are transmitted along the axon. When the sum of the dendritic activity is negative relative to the cell body, the neuron is depolarized; when it is positive, the neuron is hyperpolarized. The electroencephalogram recorded from the scalp is a measure of the summation of dendritic postsynaptic potentials rather than action potentials.
SLEEP–WAKE CYCLE: ALPHA, BETA, & GAMMA RHYTHMS
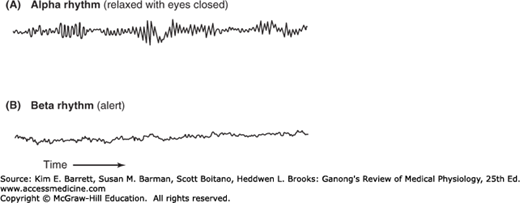
In adult humans who are awake but at rest with the mind wandering and the eyes closed, the most prominent component of the EEG is a fairly regular pattern of waves at a frequency of 8–13 Hz and amplitude of 50–100 μV when recorded from the scalp. This pattern is the alpha rhythm (Figure 14–5). It is most marked in the parietal and occipital lobes and is associated with decreased levels of attention. A similar rhythm has been observed in a wide variety of mammalian species. There are some minor variations from species to species, but in all mammals the pattern is remarkably similar (Clinical Box 14–1).
FIGURE 14–5
EEG records showing the alpha and beta rhythms. When attention is focused on something, the 8–13 Hz alpha rhythm is replaced by an irregular 13–30 Hz low-voltage activity, the beta rhythm. This phenomenon is referred to as alpha block, arousal, or the alerting response. (Used with permission from Widmaier EP, Raff H, Strang KT: Vander’s Human Physiology, 11th ed. New York, NY: McGraw-Hill; 2008.)
When attention is focused on something, the alpha rhythm is replaced by an irregular 13–30 Hz low-voltage activity, the beta rhythm (Figure 14–5). This phenomenon is called alpha block and can be produced by any form of sensory stimulation or mental concentration, such as solving arithmetic problems. Another term for this phenomenon is the arousal or alerting response because it is correlated with the aroused, alert state. It has also been called desynchronization because it represents breaking up of the obviously synchronized neural activity necessary to produce regular waves. However, the rapid EEG activity seen in the alert state is also synchronized but at a higher rate. Therefore, the term “desynchronization” is misleading. Gamma oscillations at 30–80 Hz are often seen when an individual is aroused and focuses attention on something. This is often replaced by irregular fast activity as the individual initiates motor activity in response to the stimulus.
CLINICAL BOX 14–1 Variations in the Alpha Rhythm
In humans, the frequency of the dominant EEG rhythm at rest varies with age. In infants, there is fast, beta-like activity, but the occipital rhythm is a slow 0.5–2-Hz pattern. During childhood this latter rhythm speeds up, and the adult alpha pattern gradually appears during adolescence. The frequency of the alpha rhythm is decreased by low blood glucose levels, low body temperature, low levels of adrenal glucocorticoid hormones, and high arterial partial pressure of CO2 (PaCO2). It is increased by the reverse conditions. Forced over-breathing to lower the PaCO2 is sometimes used clinically to bring out latent EEG abnormalities. The frequency and magnitude of the alpha rhythm is also decreased by metabolic and toxic encephalopathies including those due to hyponatremia and vitamin B12 deficiency. The frequency of the alpha rhythm is reduced during acute intoxication with alcohol, amphetamines, barbiturates, phenytoin, and antipsychotics. Propofol,
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree