Effects of Therapy
Diane C. Farhi
Numerous therapeutic agents are used in treating hematolymphoid disorders, and their effects are diverse. This section focuses on morphologic changes observed in the peripheral blood and bone marrow as a result of these agents.
CYTOTOXIC CHEMOTHERAPY
Cytotoxic chemotherapy usually entails a combination of several different agents, with the goal of reducing or eliminating tumor cells. Normal hematopoietic and stromal cells are also affected (Figs. 15.1, 15.2, 15.3and 15.4) (1, 2, 3, 4, 5, 6).
This type of therapy produces three morphologic stages in the bone marrow: cell death within the first week, hypocellularity at 1 to 2 weeks, and regeneration at approximately 2 weeks and thereafter. The first posttherapy marrow biopsy specimen is usually taken at 2 weeks (14 days), and thus the initial stages of cell death are often missed.
At 10 to 14 days, the marrow is usually depleted of hematopoietic cells. Aspirate smears may show eosinophilic ground substance and mucoid strands between particles. Sections show the absence of hematopoietic cells, with a relative sparing of or increase in lymphocytes and plasma cells. The marrow sinusoids are dilated. The intersinusoidal area is filled with loose fibrinous material, hemorrhage, and foamy histiocytes. Fat cells are shrunken and surrounded by eosinophilic material. The persistence of leukemic blasts at this point is a poor prognostic sign.
Regeneration begins with the appearance of small erythroid colonies in the interstitial space (sometimes as early as 5 days), followed by scattered myeloid precursors, and finally by megakaryocytes. Hematopoietic dysplasia may be present during regeneration, which should not necessarily be taken as evidence of recurrent disease. Megakaryocytes growing back after chemotherapy may show hyperplasia and dysplastic changes; in extreme cases, they resemble acute megakaryocytic leukemia (Figs. 15.5, 15.6and 15.7).
During this phase, it may be difficult to differentiate normal precursors from leukemic blasts. Immunophenotyping and cytogenetic studies may be helpful in this regard. Ultimately, repeat biopsy after the passage of time is the most reliable way to ascertain whether tumor is present. Concomitant with marrow regrowth is a recovery in peripheral blood cell counts.
Specific bone marrow changes are difficult to link with specific agents because most cases are treated with multiagent chemotherapy. Dysplastic changes, both transient and permanent (clonal), occur with many of the commonly used drugs. Megaloblastic change is particularly associated with hydroxyurea and methotrexate (Figs. 15.8 and 15.9).
DIFFERENTIATION THERAPY
Differentiation therapy is used in the treatment of granulocytic malignancies, primarily acute promyelocytic leukemia. It not only induces maturation in the malignant cells but in many instances eliminates the malignant clone without affecting normal hematopoietic cells. Differentiation therapy is frequently combined with cytotoxic therapy for greater efficacy.
All-Trans Retinoic Acid
All-trans retinoic acid (ATRA) has been successfully used in the treatment of acute promyelocytic leukemia and promyelocytic blast crisis of chronic myeloid leukemia, with both the standard t(15;17) and variant translocations (7, 8, 9, 10, 11, 12, 13, 14).
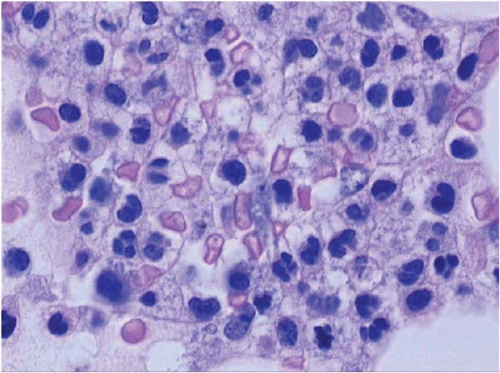
ATRA initially produces peripheral leukocytosis, followed by morphologic, cytochemical, and functional maturation of malignant promyelocytes to segmented neutrophils (Figs. 15.10, 15.11and 15.12). Maturation is evident in the peripheral blood, bone marrow, and extramedullary sites.
Although the cells mature, they retain the t(15;17) or variant translocation. The neutrophils may show retained Auer rods, nuclear filaments and blebs, and a paucity of secondary granules. Eventually, normal segmented neutrophils replace those derived from the clonal cells. As this process occurs, phagocytosis of leukemic cells by histiocytes may be noted.
Alternatively, ATRA may cause monocytic maturation of leukemic cells.
Adverse bone marrow effects of ATRA therapy include bone marrow necrosis and fibrosis, reversible with cessation of therapy, and hemophagocytic syndrome.
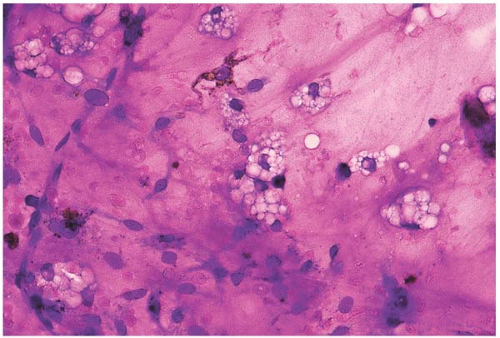 Figure 15.1 Hypoplasia after combination chemotherapy, bone marrow aspirate. A particle consists almost entirely of stromal cells and foamy histiocytes. |
 Figure 15.2 Hypoplasia after cytotoxic chemotherapy, bone marrow biopsy. Hematopoietic tissue is virtually absent, leaving stromal cells, lymphocytes, plasma cells, and histiocytes. |
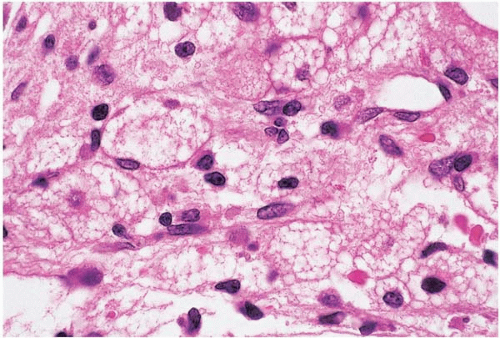 Figure 15.3 Hypoplasia after cytotoxic chemotherapy, bone marrow biopsy. Foamy histiocytes are prominent. |
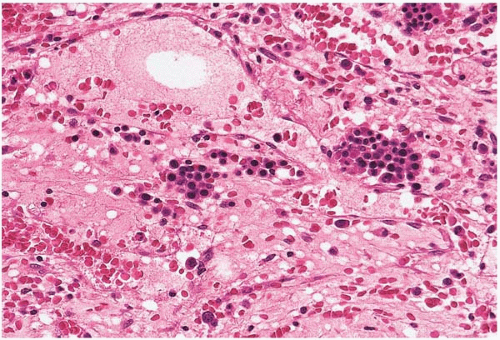 Figure 15.4 Regeneration after cytotoxic chemotherapy, bone marrow biopsy. Erythroid islands are seen in a background of stromal collapse and dilated sinusoids. |
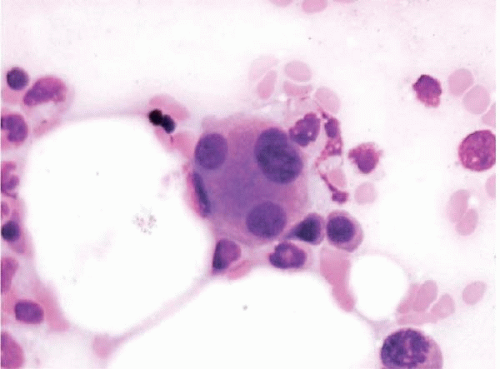 Figure 15.5 Dysplastic megakaryocyte after ATRA, bone marrow aspirate. The megakaryocyte shows small separated nuclear lobes. This effect may also be seen after standard-induction chemotherapy. |
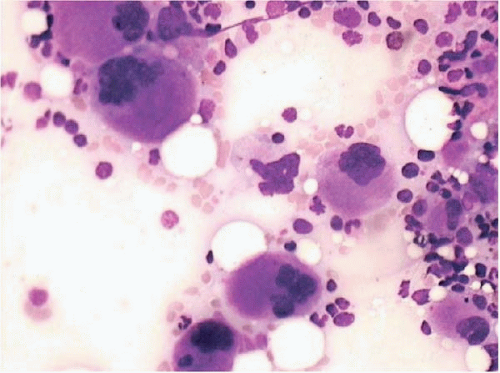 Figure 15.6 Dysplastic megakaryocytes after ATRA, bone marrow aspirate. Numerous small, atypical megakaryocytes are present. This effect may also be seen after standard-induction chemotherapy. |
 Figure 15.7 Dysplastic megakaryocytes after ATRA, bone marrow biopsy. Megakaryocytes are increased and atypical. This effect may also be seen after standard-induction chemotherapy. |
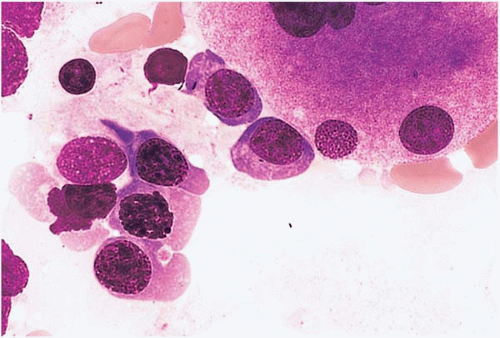 Figure 15.8 Hydroxyurea effect, peripheral blood. Erythroid precursors show megaloblastic change in this specimen from a patient with essential thrombocythemia treated with hydroxyurea. |
 Figure 15.9 Methotrexate effect, peripheral blood smear. A segmented neutrophil shows megaloblastic change. |
 Figure 15.10 ATRA effect at day 30 after therapy for acute promyelocytic leukemia, bone marrow aspirate. Neutrophils are fairly mature but atypical. |
Arsenic Trioxide
Arsenic trioxide has been used in acute promyelocytic leukemia to promote granulocytic differentiation and induce remission (15, 16, 17, 18, 19, 20, 21, 22, 23, 24, 25, 26). It has also been used in myelodysplastic syndrome and in other malignancies.
Arsenic trioxide initially induces leukopenia, followed in 15 to 20 days by leukocytosis, with maturing granulocytes. At the same time, the marrow blast count decreases, granulocytic progenitors mature, and apoptosis may be observed. Maturation is seen in both peripheral blood and marrow granulocytic precursors. Arsenic trioxide is also antiangiogenic.
Adverse effects are similar to those seen with the use of ATRA, including retinoic acid syndrome during the phase of leukocytosis and bone marrow necrosis.
HEMATOPOIETIC GROWTH FACTORS
Hematopoietic growth factors are used primarily to promote recovery of peripheral cell counts after chemotherapy and radiotherapy but have also, on occasion, helped to produce clinical remission of clonal hematopoietic disease.
Erythropoietin
Erythropoietin (EPO) has been successfully used to promote erythropoiesis in anemic patients with chronic renal failure, myelodysplastic syndrome, recovery from cytotoxic chemotherapy, normal marrow donors, and other situations (Fig. 15.13) (27, 28, 29, 30, 31, 32, 33, 34, 35).
In patients with chronic renal failure treated with EPO, the peripheral blood shows an increase in hemoglobin from an average of 6 to 10 gm’dL. The bone marrow shows increased cellularity, increased erythropoiesis with a concomitant reduction in the myeloid:erythroid ratio, and decreased intracellular iron stores.
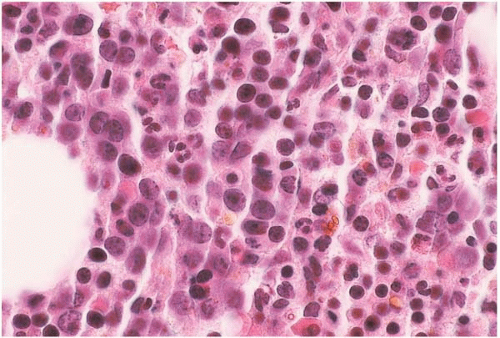 Figure 15.13 Erythropoietin effect, bone marrow biopsy. The bone marrow is hypercellular, predominantly because of proliferating erythroid precursors. |
In patients with erythroid hypoplasia caused by parvovirus B19 infection, the administration of EPO may unmask characteristic pathologic changes induced by the virus.
Hematopoietic complications of EPO therapy include rapid iron utilization, requiring concomitant iron therapy. Neutralizing antibodies to EPO may develop, resulting in intractable pure red cell aplasia. In myelodysplastic syndrome, EPO may produce accelerated disease or apparent conversion to acute leukemia, reversible upon discontinuation of the drug.
Granulocyte and Granulocyte-Macrophage Colony-Stimulating Factors
Granulocyte and granulocyte-macrophage colony-stimulating factors (G-, GM-CSFs) are used to ameliorate neutropenia and prevent infection (Figs. 15.14, 15.15, 15.16, 15.17and 15.18) (36, 37, 38, 39, 40, 41, 42, 43, 44, 45, 46, 47, 48, 49, 50, 51, 52, 53, 54, 55, 56, 57, 58).
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree