High-Yield Terms to Learn
Activated partial thromboplastin time (aPTT) test Laboratory test used to monitor the anticoagulant effect of unfractionated heparin and direct thrombin inhibitors; prolonged when drug effect is adequate Antithrombin III An endogenous anticlotting protein that irreversibly inactivates thrombin and factor Xa. Its enzymatic action is markedly accelerated by the heparins Clotting cascade System of serine proteases and substrates in the blood that provides rapid generation of clotting factors in response to blood vessel damage Glycoprotein IIb/IIIa (GPIIb/IIIa) A protein complex on the surface of platelets. When activated, it aggregates platelets primarily by binding to fibrin. Endogenous factors including thromboxane A2, ADP, and serotonin initiate a signaling cascade that activates GPIIb/IIIa
Heparin-induced thrombocytopenia (HIT) A hypercoagulable state plus thrombocytopenia that occurs in a small number of individuals treated with unfractionated heparin LMW heparins Fractionated preparations of heparin of molecular weight 2000—6000. Unfractionated heparin has a molecular weight range of 5000—30,000 Prothrombin time (PT) test Laboratory test used to monitor the anticoagulant effect of warfarin; prolonged when drug effect is adequate
Anticoagulants
Classification
Anticoagulants inhibit the formation of fibrin clots. Three major types of anticoagulants are available: heparin and related products, which must be used parenterally; direct thrombin inhibitors, which also must be used parenterally; and the orally active coumarin derivatives (eg, warfarin). Comparative properties of the heparins and warfarin are shown in Table 34-1.
TABLE 34-1 Properties of heparins and warfarin.
Property Heparins Warfarin Structure Large acidic polysaccharide polymers Small lipid-soluble molecule Route of administration Parenteral Oral Site of action Blood Liver Onset of action Rapid (minutes) Slow (days); limited by half-lives of preexisting normal factors Mechanism of action Activates antithrombin III, which proteolyzes coagulation factors including thrombin and factor Xa Impairs post-translational modification of factors II, VII, IX and X Monitoring aPTT for unfractionated heparin but not LMW heparins Prothrombin time Antidote Protamine for heparin; unfractionated protamine reversal of LMW heparins is incomplete Vitamin K1 , plasma, prothrombin complex concentrates Use Mostly acute, over days Chronic, over weeks to months Use in pregnancy Yes No
aPTT, activated partial thromboplastin time; LMW, low molecular weight.
Heparin
Chemistry
Heparin is a large sulfated polysaccharide polymer obtained from animal sources. Each batch contains molecules of varying size, with an average molecular weight of 15,000-20,000. Heparin is highly acidic and can be neutralized by basic molecules (eg, protamine). Heparin is given intravenously or subcutaneously to avoid the risk of hematoma associated with intramuscular injection.
Low-molecular-weight (LMW) fractions of heparin (eg, enoxaparin ) have molecular weights of 2000-6000. LMW heparins have greater bioavailability and longer durations of action than unfractionated heparin; thus, doses can be given less frequently (eg, once or twice a day). They are given subcutaneously. Fondaparinux is a small synthetic drug that contains the biologically active pentasaccharide present in unfractionated and LMW heparins. It is administered subcutaneously once daily.
Mechanism and Effects
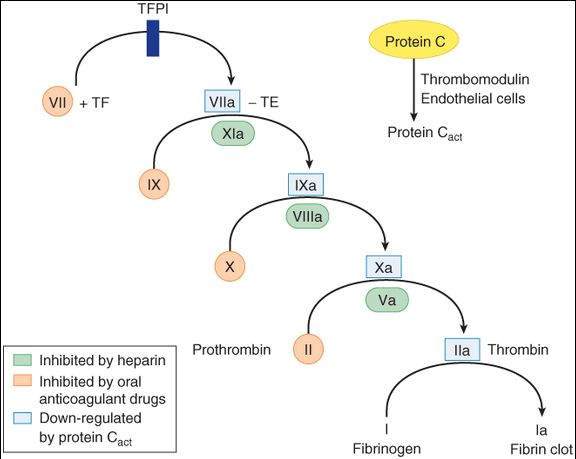
Unfractionated heparin binds to endogenous antithrombin III (ATIII) via a key pentasaccharide sequence. The heparin-ATIII complex combines with and irreversibly inactivates thrombin and several other factors, particularly factor Xa (Figure 34-1). In the presence of heparin, antithrombin III proteolyzes thrombin and factor Xa approximately 1000-fold faster than in its absence. Because it acts on preformed blood components, heparin provides anticoagulation immediately after administration. The action of heparin is monitored with the activated partial thromboplastin time (aPTT) laboratory test.
FIGURE 34-1
A model of the coagulation cascade, including its inhibition by the activated form of protein C. Tissue factor (TF) is important in initiating the cascade. Tissue factor pathway inhibitor (TFPI) inhibits the action of the VIIa-TF complex.
(Reproduced, with permission, from Katzung BG, editor: Basic & Clinical Pharmacology, 11th ed. McGraw-Hill, 2009: Fig. 34-2.)
LMW heparins and fondaparinux, like unfractionated heparin, bind ATIII. These complexes have the same inhibitory effect on factor Xa as the unfractionated heparin-ATIII complex. However, the short-chain heparin-ATIII and fondaparinux-ATIII complexes provide a more selective action because they fail to affect thrombin. The aPTT test does not reliably measure the anticoagulant effect of the LMW heparins and fondaparinux; this is a potential problem, especially in renal failure, in which their clearance may be decreased.
Clinical Use
Because of its rapid effect, heparin is used when anticoagulation is needed immediately (eg, when starting therapy). Common uses include treatment of deep vein thrombosis (DVT), pulmonary embolism, and acute myocardial infarction. Heparin is used in combination with thrombolytics for revascularization and in combination with glycoprotein IIb/IIIa inhibitors during angioplasty and placement of coronary stents. Because it does not cross the placental barrier, heparin is the drug of choice when an anticoagulant must be used in pregnancy. LMW heparins and fondaparinux have similar clinical applications.
Toxicity
Increased bleeding is the most common adverse effect of heparin and related molecules; the bleeding may result in hemorrhagic stroke. Protamine can lessen the risk of serious bleeding that can result from excessive unfractionated heparin. Protamine only partially reverses the effects of LMW heparins and does not affect the action of fondaparinux. Unfractionated heparin causes moderate transient thrombocytopenia in many patients and severe thrombocytopenia and thrombosis (heparin-induced thrombocytopenia or HIT) in a small percentage of patients who produce an antibody that binds to a complex of heparin and platelet factor 4. LMW heparins and fondaparinux are less likely to cause this immune-mediated thrombocytopenia. Prolonged use of unfractionated heparin is associated with osteoporosis.
Direct Thrombin Inhibitors
Chemistry and Pharmacokinetics
Direct thrombin inhibitors are based on proteins made by Hirudo medicinalis, the medicinal leech. Lepirudin is the recombinant form of the leech protein hirudin, while desirudin and bivalirudin are modified forms of hirudin. Argatroban is a small molecule with a short half-life. All 4 drugs are administered parenterally.
Mechanism and Effects
The protein analogs of lepirudin bind simultaneously to the active site of thrombin and to thrombin substrates. Argatroban binds solely to the thrombin-active site. Unlike the heparins, these drugs inhibit both soluble thrombin and the thrombin enmeshed within developing clots. Bivalirudin also inhibits platelet activation.
Clinical Use
Direct thrombin inhibitors are used as alternatives to heparin primarily in patients with heparin-induced thrombocytopenia. Bivalirudin also is used in combination with aspirin during percutaneous coronary angioplasty. Like unfractionated heparin, the action of these drugs is monitored with the aPTT laboratory test.
Toxicity
Like other anticoagulants, the direct thrombin inhibitors can cause bleeding. No reversal agents exist. Prolonged infusion of lepirudin can induce antibodies that form a complex with lepirudin and prolong its action, and it can induce anaphylactic reactions.
Warfarin and Other Coumarin Anticoagulants
Chemistry and Pharmacokinetics
Warfarin and other coumarin anticoagulants are small, lipid-soluble molecules that are readily absorbed after oral administration. Warfarin is highly bound to plasma proteins (>99%), and its elimination depends on metabolism by cytochrome P450 enzymes.
Mechanism and Effects
Warfarin and other coumarins interfere with the normal post-translational modification of clotting factors in the liver, a process that depends on an adequate supply of reduced vitamin K. The drugs inhibit vitamin K epoxide reductase (VKOR), which normally converts vitamin K epoxide to reduced vitamin K. The vitamin K-dependent factors include thrombin and factors VII, IX, and X (Figure 34-1). Because the clotting factors have half-lives of 8-60 h in the plasma, an anticoagulant effect is observed only after sufficient time has passed for elimination of the normal preformed factors. The action of warfarin can be reversed with vitamin K, but recovery requires the synthesis of new normal clotting factors and is, therefore, slow (6-24 h). More rapid reversal can be achieved by transfusion with fresh or frozen plasma that contains normal clotting factors. The effect of warfarin is monitored by the prothrombin time ( PT ) test.
Clinical Use
Warfarin is used for chronic anticoagulation in all of the clinical situations described previously for heparin, except in pregnant women.
Toxicity
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree