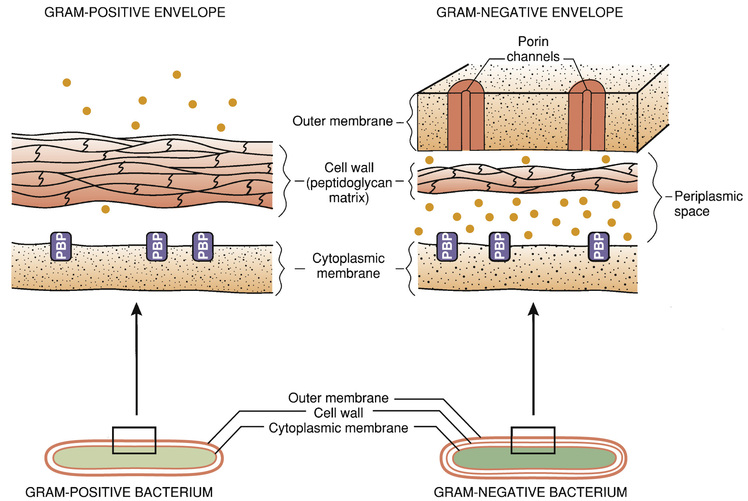
The molecular targets of the penicillins (transpeptidases, autolysins, other bacterial enzymes) are known collectively as penicillin-binding proteins (PBPs). These molecules are so named because penicillins must bind to them to produce antibacterial effects. As indicated in Fig. 69.2, PBPs are located on the outer surface of the cytoplasmic membrane. More than eight different PBPs have been identified. Of these, PBP1 and PBP3 are most critical to penicillin’s antibacterial effects. Bacteria express PBPs only during growth and division. Accordingly, because PBPs must be present for penicillins to work, these drugs work only when bacteria are growing.
Because mammalian cells lack a cell wall, and because penicillins act specifically on enzymes that affect cell wall integrity, the penicillins have virtually no direct effects on cells of the host. As a result, the penicillins are among our safest antibiotics.
Mechanisms of Bacterial Resistance
Bacterial resistance to penicillins is determined primarily by three factors: (1) inability of penicillins to reach their targets (PBPs), (2) inactivation of penicillins by bacterial enzymes, and (3) production of PBPs that have a low affinity for penicillins.
The Gram-Negative Cell Envelope
All bacteria are surrounded by a cell envelope. However, the cell envelope of gram-negative organisms differs from that of gram-positive organisms. Because of this difference, some penicillins are ineffective against gram-negative bacteria.
The cell envelope of gram-positive bacteria has only two layers: the cytoplasmic membrane and a relatively thick cell wall. Despite its thickness, the cell wall can be readily penetrated by penicillins, giving them easy access to PBPs on the cytoplasmic membrane. As a result, penicillins are generally very active against gram-positive organisms.
The gram-negative cell envelope has three layers: the cytoplasmic membrane, a relatively thin cell wall, and an additional outer membrane (see Fig. 69.2). Like the gram-positive cell wall, the gram-negative cell wall can be easily penetrated by penicillins. The outer membrane, however, is difficult to penetrate. As a result, only certain penicillins (e.g., ampicillin) are able to cross it and thereby reach PBPs on the cytoplasmic membrane.
Penicillinases (Beta-Lactamases)
Beta-lactamases are enzymes that cleave the beta-lactam ring and thereby render penicillins and other beta-lactam antibiotics inactive. Bacteria produce a large variety of beta-lactamases; some are specific for penicillins, some are specific for other beta-lactam antibiotics (e.g., cephalosporins), and some act on several kinds of beta-lactam antibiotics. Beta-lactamases that act selectively on penicillins are known as penicillinases.
Penicillinases are synthesized by gram-positive and gram-negative bacteria. Gram-positive organisms produce large amounts of these enzymes and then export them into the surrounding medium. In contrast, gram-negative bacteria produce penicillinases in relatively small amounts and, rather than exporting them to the environment, secrete them into the periplasmic space (see Fig. 69.2).
The genes that code for beta-lactamases are located on chromosomes and on plasmids (extrachromosomal DNA). The genes on plasmids may be transferred from one bacterium to another, thereby promoting the spread of penicillin resistance.
Transfer of resistance is of special importance with Staphylococcus aureus. When penicillin was first introduced in the early 1940s, all strains of S. aureus were sensitive. However, by 1960, as many as 80% of S. aureus isolates in hospitals displayed penicillin resistance. Fortunately, a penicillin derivative (methicillin) that has resistance to the actions of beta-lactamases was introduced at this time. To date, no known strains of S. aureus produce beta-lactamases capable of inactivating methicillin or related penicillinase-resistant penicillins (although some strains are resistant to these drugs for other reasons).
Altered Penicillin-Binding Proteins
Certain bacterial strains, known collectively as methicillin-resistant Staphylococcus aureus (MRSA), have a unique mechanism of resistance: production of PBPs with a low affinity for penicillins and all other beta-lactam antibiotics. MRSA developed this ability by acquiring genes that code for low-affinity PBPs from other bacteria.
Methicillin-Resistant Staphylococcus aureus
S. aureus is a gram-positive bacterium that often colonizes the skin and nostrils of healthy people. Infection usually involves the skin and soft tissues, causing abscesses, boils, cellulitis, and impetigo. However, more serious infections can also develop, including infections of the lungs and bloodstream, which can be fatal.
Like other pathogens, S. aureus has developed resistance over the years. When penicillins were introduced in the 1940s, all strains of S. aureus were susceptible. However, penicillin-resistant strains quickly emerged, owing to bacterial production of penicillinases. In 1959 this resistance was overcome with methicillin, the first penicillinase-resistant penicillin. Unfortunately, by 1968, strains resistant to methicillin had emerged. These highly resistant bacteria, known as MRSA, are resistant not only to methicillin (now obsolete) but also to all penicillins and all cephalosporins as well. The basis of MRSA resistance is acquisition of genes that code for penicillin-binding proteins that have very low affinity for penicillins and cephalosporins. Resistant strains were initially limited to health care facilities but are now found in the community as well.
In the United States MRSA is a serious public health problem. In 2014 it was estimated that 75,309 infections were caused by MRSA. Not only does MRSA increase mortality, it also increases costs: treating hospitalized MRSA patients costs about $35,000, compared with $14,000 for patients with methicillin-sensitive infections. Fortunately, the MRSA news isn’t all bad. For one thing, although MRSA infections are now common, most patients can be cured. Also, rates of MRSA infection among hospitalized patients are now falling, after rising steadily for many years. MRSA infections that began in hospitals declined 54% between 2005 and 2011, with 30,800 fewer severe infections.
There are two distinct types of MRSA, referred to as health care−associated MRSA (HCA-MRSA) and community-associated MRSA (CA-MRSA). Of the two, HCA-MRSA is more prevalent (80% vs. 20%) and emerged earlier (1968 vs. 1981). Also, HCA-MRSA infection is generally more serious and harder to treat. Molecular typing indicates that HCA-MRSA and CA-MRSA are genetically distinct strains, known as USA100 and USA300, respectively.
Health Care−Associated MRSA
Methicillin resistance in S. aureus was first reported in isolates from hospitalized patients in 1968. For most of the next four decades, the prevalence of HCA-MRSA among hospitalized patients climbed steadily, reaching 85% of all invasive S. aureus infections by 2004.
Although many infections with HCA-MRSA surface in the community, nearly all occur in people who had been exposed to a health care facility within the prior year, indicating that acquisition of the infection probably occurred in a health care setting—not out in the community. Transmission of HCA-MRSA is usually through person-to-person contact, very often between health care workers and patients. Risk factors for acquiring HCA-MRSA include advanced age, recent surgery or hospitalization, dialysis, treatment in an intensive care unit, prolonged antibiotic therapy, an indwelling catheter, and residence in a long-term care facility.
The treatment of HCA-MRSA infection is addressed at length in a guideline—Clinical Practice Guidelines by the Infectious Diseases Society of America for the Treatment of Methicillin-Resistant Staphylococcus aureus Infections in Adults and Children. The guideline stresses the importance of selecting drugs based on the site of the infection, age of the patient, and drug sensitivity of the pathogen. For complicated skin and soft tissue infections in adults, the preferred drugs are intravenous (IV) vancomycin, linezolid [Zyvox], daptomycin [Cubicin], telavancin [Vibativ], clindamycin, and ceftaroline [Teflaro]. IV vancomycin is the preferred drug for children. For bacteremia or endocarditis in adults or children, IV vancomycin and daptomycin are drugs of choice. Preferred drugs for pneumonia in adults and children are IV vancomycin, linezolid, and clindamycin. Because most strains of MRSA are multidrug resistant, many other antibiotics are ineffective, including tetracyclines, clindamycin, trimethoprim/sulfamethoxazole, and beta-lactam agents (except ceftaroline).
Community-Associated MRSA
Infection with CA-MRSA, first reported in 1981, is caused by staphylococcal strains that are genetically distinct from HCA-MRSA. For example, most strains of CA-MRSA carry a gene for Panton-Valentine leukocidin (a cytotoxin that causes necrosis), whereas HCA-MRSA strains do not. Many people are now asymptomatic carriers of CA-MRSA. In fact, between 20% and 30% of the population is colonized, typically on the skin and in the nostrils.
Infection with CA-MRSA is generally less dangerous than with HCA-MRSA but is more dangerous than with methicillin-sensitive S. aureus. In most cases, CA-MRSA causes mild infections of the skin and soft tissues, manifesting as boils, impetigo, and so forth. However, CA-MRSA can also cause more serious infections, including necrotizing fasciitis, severe necrotizing pneumonia, and severe sepsis. Fortunately, these invasive infections are relatively rare. On the other hand, infections of the skin and soft tissues are now common, with CA-MRSA accounting for more than 50% of the S. aureus isolates from these sites.
CA-MRSA transmission is by skin-to-skin contact and by contact with contaminated objects, including frequently touched surfaces, sports equipment, and personal items (e.g., razors). In contrast to HCA-MRSA infection, CA-MRSA infection is seen primarily in young, healthy people with no recent exposure to health care facilities. Individuals at risk include athletes in contact sports (e.g., wrestling), men who have sex with men, and people who live in close quarters, such as family members, child care clients, prison inmates, military personnel, and college students.
Several measures can reduce the risk for CA-MRSA transmission. Topping the list is good hand hygiene—washing with soap and water or applying an alcohol-based sanitizer. Other measures include showering after contact sports, cleaning frequently touched surfaces, keeping infected sites covered, and not sharing towels and personal items.
Treatment depends on infection severity. For boils, small abscesses, and other superficial infections, surgical drainage may be all that is needed. For more serious infections, drugs may be indicated. Preferred agents are trimethoprim/sulfamethoxazole, minocycline, doxycycline, and clindamycin. Alternative drugs—vancomycin, daptomycin, and linezolid—should be reserved for severe infections and treatment failures. To eradicate the carrier state, intranasal application of a topical antibiotic—mupirocin or retapamulin—can be effective. Like HCA-MRSA, CA-MRSA does not respond to beta-lactam antibiotics, except ceftaroline.
Chemistry
All of the penicillins are derived from a common nucleus: 6-aminopenicillanic acid. This nucleus contains a beta-lactam ring joined to a second ring. The beta-lactam ring is essential for antibacterial actions. Properties of individual penicillins are determined by additions made to the basic nucleus. These modifications determine (1) affinity for PBPs, (2) resistance to penicillinases, (3) ability to penetrate the gram-negative cell envelope, (4) resistance to stomach acid, and (5) pharmacokinetic properties.
Classification
The most useful classification of penicillins is based on antimicrobial spectrum. When classified this way, the penicillins fall into four major groups: (1) narrow-spectrum penicillins that are penicillinase sensitive, (2) narrow-spectrum penicillins that are penicillinase resistant (antistaphylococcal penicillins), (3) broad-spectrum penicillins (aminopenicillins), and (4) extended-spectrum penicillins (antipseudomonal penicillins). Table 69.1 lists the members of each group and their principal target organisms.
TABLE 69.1
Classification of the Penicillins
| Penicillin Class | Drug | Clinically Useful Antimicrobial Spectrum |
| Narrow-spectrum penicillins: penicillinase sensitive | Penicillin G Penicillin V | Streptococcus spp., Neisseria spp., many anaerobes, spirochetes, others |
| Narrow-spectrum penicillins: penicillinase resistant (antistaphylococcal penicillins) | Nafcillin Oxacillin Dicloxacillin | Staphylococcus aureus |
| Broad-spectrum penicillins (aminopenicillins) | Ampicillin Amoxicillin | Haemophilus influenzae, Escherichia coli, Proteus mirabilis, enterococci, Neisseria gonorrhoeae |
| Extended-spectrum penicillin (antipseudomonal penicillin) | Piperacillin | Same as broad-spectrum penicillins plus Pseudomonas aeruginosa, Enterobacter spp., Proteus (indole positive), Bacteroides fragilis, many Klebsiella spp. |
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


