Insulin Preparations Insulin lispro (short duration, rapid acting) Regular insulin (short duration, slower acting) NPH insulin (intermediate duration) Insulin glargine (long duration) Biguanide Metformin Sulfonylurea Glyburide Meglitinide (Glinide) Repaglinide Thiazolidinedione (Glitazone) Pioglitazone Alpha-Glucosidase Inhibitor Acarbose Gliptin (Dipeptidyl Peptidase-4 Inhibitor) Sitagliptin Sodium-Glucose Cotransporter 2 Inhibitor Canagliflozin Incretin Mimetic Exenatide
Insulin
Insulin is used to treat all patients with type 1 diabetes and many patients with type 2 diabetes. Our discussion of insulin is divided into three sections: physiology, preparations and administration, and therapeutic use.
Physiology
Structure
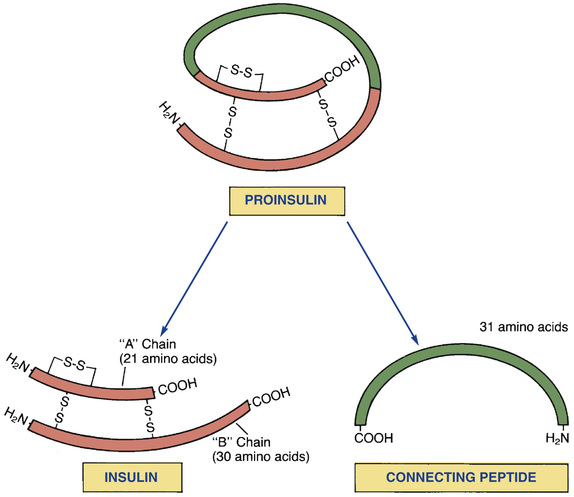
The structure of insulin is shown in Fig. 46.1. As indicated, insulin consists of two amino acid chains: the “A” (acidic) chain and the “B” (basic) chain. The A and B chains are linked to each other by two disulfide bridges.
Biosynthesis
Insulin is synthesized in the pancreas by beta cells within the islets of Langerhans. The immediate precursor of insulin is called proinsulin.
Proinsulin consists of insulin itself plus a peptide loop that runs from the A chain to the B chain. This loop is named connecting peptide or C-peptide. In the final step of insulin synthesis, C-peptide is enzymatically clipped from the proinsulin molecule.
Measurement of plasma C-peptide levels offers a way to assess residual capacity for insulin synthesis. Because commercial insulin preparations lack C-peptide, and because endogenous C-peptide is only present as a byproduct of insulin biosynthesis, the presence of C-peptide in the blood indicates the pancreas is still producing some insulin of its own.
Secretion
The principal stimulus for insulin release is a rise in blood glucose, and the most common cause of glucose elevation is eating a meal, especially one rich in carbohydrates. Under normal conditions, there is tight coupling between rising levels of blood glucose and increased secretion of insulin. Insulin release may also be triggered by amino acids, fatty acids, ketone bodies, and gut hormones such as GLP-1 (more on this later).
The sympathetic nervous system provides additional control of release. Activation of beta2-adrenergic receptors in the pancreas promotes secretion of insulin. Conversely, activation of alpha-adrenergic receptors in the pancreas inhibits insulin release. Of the two modes of regulation, activation of beta receptors is more important.
Metabolic Actions
The metabolic actions of insulin are primarily anabolic (i.e., conservative, constructive). Insulin promotes conservation of energy and buildup of energy stores, such as glycogen. The hormone also promotes cell growth and division.
Insulin acts in two ways to promote anabolic effects. First, it stimulates cellular transport (uptake) of glucose, amino acids, nucleotides, and potassium. Second, insulin promotes synthesis of complex organic molecules. Under the influence of insulin and other factors, glucose is converted into glycogen, amino acids are assembled into proteins, and fatty acids are incorporated into triglycerides. The principal metabolic actions of insulin are shown in Table 46.5.
TABLE 46.5
Metabolic Actions of Insulin
| Substance Affected | Insulin Action | Site of Action |
| Carbohydrates | ↑ Glucose uptake | Muscle, adipose tissue |
| ↑ Glucose oxidation | Muscle | |
↑ Glucose storage ↑ Glycogen synthesis ↓ Glycogenolysis | Muscle, liver | |
| Gluconeogenesis* | Liver | |
| Amino acids and proteins | ↑ Amino acid uptake | Muscle |
| ↓ Amino acid release | Muscle | |
| ↑ Protein synthesis | Muscle | |
| Lipids | ↑ Triglyceride synthesis | Adipose tissue |
| ↓ Release of FFA and glycerol | Adipose tissue | |
| ↓ Oxidation of FFA to ketoacids† | Liver |
Metabolic Consequences of Insulin Deficiency
Insulin deficiency puts the body into a catabolic mode. Hence, in the absence of insulin, glycogen is converted into glucose, proteins are degraded into amino acids, and fats are converted to glycerol (glycerin) and free fatty acids. These catabolic effects contribute to the signs and symptoms of diabetes. Note that the catabolic effects resulting from insulin deficiency are opposite to the anabolic effects when insulin levels are normal.
Insulin deficiency promotes hyperglycemia by three mechanisms: (1) increased glycogenolysis, (2) increased gluconeogenesis, and (3) reduced glucose utilization. Glycogenolysis, by definition, generates free glucose by breaking down glycogen. The raw materials that allow increased gluconeogenesis are the amino acids and fatty acids produced by metabolic breakdown of proteins and fats. Reduced glucose utilization occurs because insulin deficiency decreases cellular uptake of glucose and decreases conversion of glucose to glycogen.
Preparations and Administration
There are many insulin preparations or formulations. Major differences concern time course, appearance (clear or cloudy), concentration, and route of administration. Because of these differences, insulin preparations cannot be used interchangeably. In fact, if a patient is given the wrong preparation, the consequences can be dire. Unfortunately, medication errors with insulins remain all too common, which explains why insulin appears on all lists of “high-alert” agents.
Sources of Insulin
All forms of insulin currently manufactured in the United States are produced using recombinant DNA technology. Some products, referred to as human insulin, are identical to insulin produced by the human pancreas. Other products, referred to as human insulin analogs, are modified forms of human insulin. The analogs have the same pharmacologic actions as human insulin but have different time courses.
Types of Insulin
There are seven types of insulin: “natural” insulin (also known as regular insulin or native insulin) and six modified insulins. Three of the modified insulins—insulin lispro, insulin aspart, and insulin glulisine—act more rapidly than regular insulin but have a shorter duration of action. The other modified insulins act more slowly than regular insulin but have a longer duration. Two processes are used to prolong insulin effects: (1) complexing natural insulin with a protein and (2) altering the insulin molecule itself. When the insulin molecule has been altered, we refer to the product as a human insulin analog.
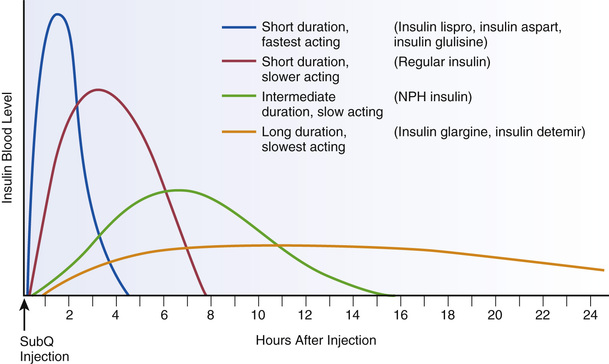
When classified according to time course, insulin preparations fall into three major groups: short duration, intermediate duration, and long duration (Table 46.6). The short-duration insulins can be subdivided into two groups: rapid acting (insulin lispro, insulin aspart, and insulin glulisine) and slower acting (regular or “natural” insulin). Time courses for different insulin types are shown in Fig. 46.2. Selected properties of insulin types are shown in Table 46.7.
TABLE 46.6
Types of Insulin: Time Course of Action After Subcutaneous Injection
| Time Course | ||||
| Generic Name | Trade Name | Onset (min) | Peak (hr) | Duration (hr) |
| SHORT DURATION: RAPID ACTING | ||||
| Insulin lispro | Humalog | 15–30 | 0.5–2.5 | 3–6 |
| Insulin aspart | NovoLog | 10–20 | 1–3 | 3–5 |
| Insulin glulisine | Apidra | 10–15 | 1–1.5 | 3–5 |
| SHORT DURATION: SLOWER ACTING | ||||
| Regular insulin | Humulin R, Novolin R | 30–60 | 1–5 | 6–10 |
| INTERMEDIATE DURATION | ||||
| NPH insulin | Humulin N, Novolin N | 60–120 | 6–14 | 16–24 |
| LONG DURATION | ||||
| Insulin glargine | Lantus | 70 | None* | 18–24 |
| Insulin detemir | Levemir | 60–120 | 12–24 | Varies† |
| ULTRALONG DURATION | ||||
| Insulin degludec | Tresiba | 60 | None* | 42 |
TABLE 46.7
Properties of Insulin Types
| Generic Name [Trade Name] | Class | Rx or OTC | Strength | Appearance | Route | Administration Options |
| SHORT DURATION: RAPID ACTING | ||||||
| Insulin lispro [Humalog] | HA | Rx | U-100 | Clear | SubQ, IV | SubQ inj: within 15 min before or just after meals SubQ inf: continuous, with bolus just before meals IV: approved route, but rarely used |
| Insulin aspart [NovoLog] | HA | Rx | U-100 | Clear | SubQ, IV | SubQ inj: 5–10 min before meals SubQ inf: continuous, with bolus 5–10 min before meals IV: approved route, but rarely used |
| Insulin glulisine [Apidra] | HA | Rx | U-100 | Clear | SubQ, IV | SubQ inj: within 15 min before meals or within 20 min after SubQ inf: continuous, with bolus 15–20 min before meals IV: approved route, but rarely used |
| SHORT DURATION: SLOWER ACTING | ||||||
| Regular insulin [Humulin R, Novolin R] | H | OTC* | U-100, U-500 | Clear | SubQ, IV, IM | SubQ inj: 30 min before meals SubQ inf: continuous, with bolus 20–30 min before meals IV: for emergencies and glycemic management in the inpatient setting (never use U-500 IV) IM: approved route, but rarely used |
| INTERMEDIATE DURATION | ||||||
| NPH insulin [Humulin N, Novolin N] | H | OTC | U-100 | Cloudy | SubQ | SubQ inj: twice daily at the same times each day; gently agitate before use |
| LONG DURATION | ||||||
| Insulin glargine [Lantus] | HA | Rx | U-100 | Clear | SubQ | SubQ inj: once or twice daily at the same time each day |
| Insulin detemir [Levemir] | HA | Rx | U-100 | Clear | SubQ | SubQ inj: once or twice daily at the same time each day |
| ULTRALONG DURATION | ||||||
Insulin degludec [Tresiba] | HA | Rx | U-100, U-200 | Clear | SubQ | SubQ inj: once daily at the same time each day |
Short Duration: Rapid Acting
Short-duration insulins are administered in association with meals to control the postprandial rise in blood glucose. To provide glycemic control between meals and at night, short-acting insulins must be used in conjunction with an intermediate- or long-acting agent in people with type 1 diabetes. All three of the rapid-acting insulins are formulated as clear solutions, and all three require a prescription. For routine therapy, all three are given by the subcutaneous (subQ) route.
Insulin Lispro.
Insulin lispro [Humalog] is a rapid-acting analog of regular insulin. Effects begin within 15 to 30 minutes of subQ injection and persist for 3 to 6 hours. Insulin lispro acts faster than regular insulin but has a shorter duration of action. Because of its rapid onset, insulin lispro can be administered immediately before eating, or even after eating. In contrast, regular insulin is generally administered 30 to 60 minutes before meals. The usual route for insulin lispro is subQ by injection or use of an insulin pump. Insulin lispro (100 units/mL) is commercially available in 10-mL vials and as 3-mL prefilled pens.
The structure of insulin lispro is nearly identical to that of natural insulin. The only difference is that the positions of two amino acids have been switched. Because of this switch, molecules of insulin lispro aggregate less than do molecules of regular insulin, which explains why insulin lispro acts more rapidly.
Insulin Aspart.
Insulin aspart [NovoLog] is an analog of human insulin with a rapid onset (10–20 minutes) and short duration (3–5 hours). Insulin aspart is very similar to insulin lispro.
Insulin aspart (100 units/mL) is supplied in 10-mL vials and 3-mL prefilled pens. Dosing is almost always done by subQ injection or subQ infusion. Because insulin aspart acts rapidly, injections should be made 5 to 10 minutes before meals.
Insulin Glulisine.
Like insulin lispro and insulin aspart, insulin glulisine [Apidra] is a synthetic analog of natural human insulin with a rapid onset (10–15 minutes) and short duration (3–5 hours). Owing to its rapid onset, the drug should be administered close to the time of eating. Administration is almost always by subQ injection or continuous subQ infusion. Insulin glulisine (100 units/mL) is available in 10-mL vials and as 3-mL prefilled insulin pens.
Short Duration: Slower Acting
Regular Insulin Injection.
Regular insulin [Humulin R, Novolin R] is unmodified human insulin. The product has four approved routes: subQ injection, subQ infusion, IM injection (used rarely), and oral inhalation.
For routine treatment of diabetes, regular insulin can be (1) injected before meals to control postprandial hyperglycemia and (2) infused subcutaneously to provide basal glycemic control. After subQ injection, molecules of regular insulin form small aggregates at the injection site. As a result, absorption is slightly delayed. Effects begin in 30 to 60 minutes, peak in 1 to 5 hours, and last up to 10 hours. Onset is slower than with the rapid-acting insulins and faster than with the longer acting insulins. Because of this delay, most people using insulin pumps use a rapid-acting insulin analog instead of regular insulin.
Regular insulin is supplied as a clear solution. Two concentrations are available: U-100 (100 units/mL) and U-500 (500 units/mL). Regular insulin [Humulin R] is the only type available in a U-500 strength. U-100 preparations are used by most patients. The U-500 concentration is reserved for patients with extreme insulin resistance. Care should also be taken when using U-500 insulin because insulin syringes are calibrated to be used with U-100 products. Extra caution and education are critical when working with patients using U-500 insulin. Except for the U-500 formulation, all formulations of regular insulin are available without prescription.
Intermediate Duration
Neutral Protamine Hagedorn (NPH) Insulin Suspension.
NPH insulin [Humulin N, Novolin N], also known as isophane insulin, is prepared by conjugating regular insulin with protamine (a large protein). The presence of protamine decreases the solubility of NPH insulin and thus delays absorption. As a result, onset of action is delayed and duration of action is extended. Because onset is delayed, NPH insulin cannot be administered at mealtime to control postprandial hyperglycemia. Rather, the drug is injected twice or three times daily to provide glycemic control between meals and during the night. Of the longer acting insulins in current use, NPH insulin is the only one suitable for mixing with short-acting insulins. Because protamine is a foreign protein, allergic reactions are possible. NPH insulins are supplied as cloudy suspensions that must be agitated before administration. Administration is by subQ injection only. Like regular insulin, NPH insulins are available without prescription. NPH insulin (100 units/mL) is available in 10-mL vials and in 3-mL prefilled insulin pens.
Long Duration
Insulin Glargine.
Insulin glargine [Lantus] is a modified human insulin with a prolonged duration of action (up to 24 hours). The drug is indicated for once-daily subQ dosing to treat adults and children with type 1 diabetes and adults with type 2 diabetes. That being said, some patients require twice-daily administration to achieve a full 24 hours of basal coverage. Dosing may be done any time of day (morning, afternoon, or evening) but should be done at the same time every day, if possible.
Insulin glargine differs from natural human insulin by four amino acids. Hence, when injected subcutaneously, it forms microprecipitates that slowly dissolve and thereby release insulin glargine in small amounts over an extended time. In contrast to other long-acting insulins (e.g., insulin detemir), which cause blood levels to rise to a peak and then fall to a trough, insulin glargine achieves blood levels that are relatively steady.
Insulin glargine is supplied as a clear solution in 10-mL vials containing 100 units/mL, and as a prefilled SoloStar Pen. The drug should not be mixed with other insulins.
Insulin Detemir.
Insulin detemir [Levemir] is a human insulin analog with a slow onset and dose-dependent duration of action. At low doses (0.2 units/kg), effects persist about 12 hours. At higher doses (0.4 units/kg), effects persist for up to 20 to 24 hours. Because of its slow onset and prolonged duration, insulin detemir is used to provide basal glycemic control. It is not given before meals to control postprandial hyperglycemia. Compared with NPH insulin, insulin detemir has a slower onset and longer duration.
Insulin detemir is supplied as a clear, colorless solution (100 units/mL) in 10-mL vials and as a 3-mL FlexPen. Dosing is done once or twice daily by subQ injection. Insulin detemir should not be mixed with other insulins and must not be given intravenously. The drug is available by prescription only.
Ultralong Duration
Insulin Degludec.
Insulin degludec [Tresiba] is the only human insulin analog with ultralong duration of action. Effects of insulin degludec persist for up to 42 hours. Because of its prolonged duration, insulin degludec is used to provide basal glycemic control. Similarly to insulin glargine, insulin degludec does not have a peak.
Insulin degludec is supplied as a clear, colorless solution in two concentrations of FlexTouch pens (100 units/mL and 200 units/mL). Dosing is done once daily by subQ injection. Insulin degludec should not be mixed with other insulins and must not be given intravenously. The drug is available by prescription only.
Concentration
In the United States insulin is available in two concentrations: 100 units/mL (U-100) and 500 units/mL (U-500). Preparations containing 40 units/mL are available in other countries but not in the United States. U-100 insulins are employed for routine replacement therapy. All insulin types are available in U-100 formulations. Only one product—the Humulin R brand of regular insulin—is formulated in the U-500 strength. This product, which is available from the manufacturer by special request, is reserved for emergencies and for patients with severe insulin resistance, generally defined as needing more than 200 units/day.
Subcutaneous Infusion
Portable Insulin Pumps.
These computerized devices deliver a basal infusion of insulin (regular, lispro, aspart, or glulisine) plus bolus doses before each meal. In other words, the pump uses only one type of insulin for both basal and mealtime coverage. The basal infusion is usually about 1 unit/hour and can be programmed to match the patient’s metabolic requirements. Basal rates can even be adjusted to different rates throughout the day, depending on the individualized needs of the patient, and are adjustable in some pumps up to 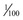 of a unit per hour. Mealtime boluses are calculated to match carbohydrate intake and can be adjusted to within
of a unit per hour. Mealtime boluses are calculated to match carbohydrate intake and can be adjusted to within 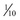 of a unit. The pumps are about the size of a small cell phone, weigh only 4 ounces, and are worn on the belt or in a pocket. An infusion set delivers insulin from the pump to a subcutaneous catheter, usually located on the abdomen. The infusion set should be replaced every 1 to 3 days, at which time the catheter is moved to a new infusion site (at least 1 inch away from the old one). Because the pump delivers short-acting insulin, insulin levels will drop quickly if the pump is removed. Accordingly, the pump should remain in place most of the day. However, it can be removed for 1 to 2 hours on special occasions. External insulin pumps cost between $3000 and $5000. Infusion sets, insulin, and glucose monitoring materials add another $300 or more per month to the bill. Aside from expense, the main drawback of the pumps is delivery of too little insulin owing to formation of insulin microdeposits within the tubing.
of a unit. The pumps are about the size of a small cell phone, weigh only 4 ounces, and are worn on the belt or in a pocket. An infusion set delivers insulin from the pump to a subcutaneous catheter, usually located on the abdomen. The infusion set should be replaced every 1 to 3 days, at which time the catheter is moved to a new infusion site (at least 1 inch away from the old one). Because the pump delivers short-acting insulin, insulin levels will drop quickly if the pump is removed. Accordingly, the pump should remain in place most of the day. However, it can be removed for 1 to 2 hours on special occasions. External insulin pumps cost between $3000 and $5000. Infusion sets, insulin, and glucose monitoring materials add another $300 or more per month to the bill. Aside from expense, the main drawback of the pumps is delivery of too little insulin owing to formation of insulin microdeposits within the tubing.
Implantable Insulin Pumps.
These devices are surgically implanted in the abdomen and deliver insulin either intraperitoneally or intravenously. Like external pumps, internal pumps deliver a basal insulin infusion plus bolus doses with meals. Insulin delivery is adjusted by external telemetry. Compared with multiple daily injections, pumps produce superior glycemic control, cause less hypoglycemia and weight gain, and can improve quality of life. As with external pumps, delivery of insulin can be impeded by formation of insulin microprecipitates. Implantable pumps are experimental and not yet available for general use.
Inhalation
Inhalation of insulin is an attractive alternative to needle-based dosing. In clinical trials, patient satisfaction with inhaled insulin has been much higher than with insulin injections. One delivery system is currently available for insulin inhalation—Afrezza. This inhaled mealtime insulin product provides good glycemic control with a relatively low incidence of hypoglycemia—and has demonstrated little or no effect on pulmonary function in studies to date. Even though it has not demonstrated effects on pulmonary function, Afrezza is known to cause bronchospasm in patients with chronic lung disease. Therefore there is a Risk Evaluation and Mitigation Strategy (REMS) program in place to protect patients with chronic obstructive pulmonary disease or asthma. Before prescribing Afrezza, the provider should obtain a detailed medical history, physical examination, and spirometry.
Storage
Insulin in unopened vials should be stored under refrigeration until needed. Vials should not be frozen. When stored unopened under refrigeration, insulin can be used up to the expiration date on the vial.
The vial in current use can be kept at room temperature for up to 1 month without significant loss of activity. Direct sunlight and extreme heat must be avoided. Partially filled vials should be discarded after several weeks if left unused. Injecting insulin stored at room temperature causes less pain than injecting cold insulin and reduces the risk for lipodystrophy.
Mixtures of insulin prepared in vials are stable for 1 month at room temperature and for 3 months under refrigeration.
Mixtures of insulin in prefilled syringes (plastic or glass) should be stored in a refrigerator, where they will be stable for at least 1 week and perhaps 2 weeks. The syringe should be stored vertically with the needle pointing up to avoid clogging the needle. Before administration, the syringe should be agitated gently to resuspend the insulin.
Therapeutic Use
Indications
The principal indication for insulin is diabetes mellitus. Insulin is required by all patients with type 1 diabetes and by many patients with type 2 diabetes. In fact, most of the insulin sold is used by people with type 2 diabetes—largely because type 2 diabetes accounts for 90% to 95% of all cases of diabetes.
Insulin Therapy of Diabetes
Insulin is given to all patients who have type 1 diabetes and to many who have type 2 diabetes. In addition, insulin is the preferred drug to manage gestational diabetes. In treating these disorders, the objective is to prevent complications by keeping blood glucose within an acceptable range. When therapy is successful, both hyperglycemia and hypoglycemia are minimized, and the long-term complications of diabetes are avoided.
Dosage
To achieve optimal glucose control, insulin dosage must be closely matched with insulin needs. If carbohydrate intake is increased, insulin dosage must be increased, too. When a meal is missed or is low in carbohydrates, or when physical activity levels increase, the dosage of insulin must be decreased. Dosing requires additional adjustments to meet specialized needs. For example, insulin needs are increased by infection, stress, obesity, the adolescent growth spurt, and pregnancy after the first trimester. Conversely, insulin needs are decreased by exercise and during the first trimester of pregnancy. To ensure that insulin dosage is coordinated with insulin requirements, the patient and the health care team must work together to establish an integrated program of nutrition, exercise, insulin replacement therapy, and appropriate blood glucose monitoring.
Total daily dosages may range from 0.1 unit/kg body weight to more than 2.5 units/kg. For patients with type 1 diabetes, initial dosages typically range from 0.5 to 0.6 units/kg/day. For patients with type 2 diabetes, initial dosages typically range from 0.2 to 0.6 units/kg/day.
Dosing Schedules
The schedule of insulin administration helps determine the extent to which glucose control can be achieved. Three dosing schedules are compared here. These example regimens include use of (1) a twice-daily premixed insulin regimen, (2) intensive basal/bolus strategy, and (3) continuous subcutaneous insulin infusion (CSII). Although three example regimens are discussed, it should be noted that practitioners can use available insulin products in a number of ways and combinations to meet patient-specific needs and treatment goals.
Twice-Daily Premixed Regimen.
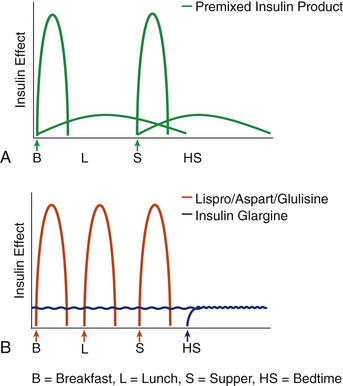
There are several premixed insulin products on the market. As shown in Fig. 46.4A, a twice-daily regimen of such a premixed insulin product can be used to provide both basal and prandial insulin coverage. The advantage of this strategy is that patients only have to give two injections per day. A disadvantage, however, is that if given with breakfast and dinner, there is no mealtime coverage at lunch. Additionally, using a fixed combination does not allow for adjustments of the long-acting or short-acting insulin individually; if the dose is changed, both components are altered.
Intensive Basal/Bolus Strategy.
For patients with type 1 diabetes, an intensive basal/bolus strategy is often used. As shown in Fig. 46.4B, this strategy involves the use of a long-acting insulin (such as insulin glargine [Lantus]) in addition to a short-acting insulin (such as regular insulin, insulin aspart, insulin lispro, or insulin glulisine). This insulin dosing strategy allows for very good basal coverage and the ability to dose a short-acting insulin with each meal and as needed to cover snacks or elevated blood glucose levels.
Continuous Subcutaneous Insulin Infusion.
CSII is accomplished using a portable infusion pump connected to an indwelling subcutaneous catheter. Four types of insulin may be used: regular, lispro, aspart, and glulisine. To provide a basal level of insulin, the pump is set to infuse insulin continuously at a slow but steady rate. To accommodate insulin needs created by eating, the pump is triggered manually to provide a bolus dose matched in size to the carbohydrate content of each meal. Hence, CSII can adapt to altered insulin needs. Although the use of CSII allows for ease of administration, the use of frequent SMBG is essential to achieve optimal glycemic control. Infusion pumps were discussed earlier in the section on “Subcutaneous Infusion.”
Achieving Optimal Glucose Control
As we have seen, the primary requirement for achieving tight glucose control is a method of insulin delivery that permits dosage adjustments that accommodate ongoing variations in insulin needs. Intensive basal/bolus therapy and CSII meet this criterion. In addition to an adaptable method of insulin delivery, achieving tight glucose control requires the following:
• Careful attention to all elements of the treatment program (diet, exercise, insulin replacement therapy)
• Self-monitoring of blood glucose in concordance with the patient’s individualized management plan
• A high degree of patient motivation
Tight glucose control cannot be achieved without the informed participation of the patient. Accordingly, patients must receive thorough instruction on the following:
• The importance of optimal glucose control
• The major components of the treatment routine (insulin replacement, SMBG, diet, exercise)
• Procedures for purchasing insulin, syringes, and needles
• The importance of avoiding arbitrary changes between insulins from different manufacturers
• Procedures for mixing insulins (if applicable)
• Calculation of dosage adjustments
• Techniques of insulin administration
In the final analysis, responsibility for managing diabetes rests with the patient. The health care team can design a treatment program and provide education and guidance. However, optimal glucose control can only be achieved if the patient is actively involved in his or her own therapy.
Complications of Insulin Treatment
Hypoglycemia
Hypoglycemia (blood glucose below 70 mg/dL) occurs when insulin levels exceed insulin needs. A major cause of insulin excess is overdose. Imbalance between insulin levels and insulin needs can also result from reduced intake of food, vomiting and diarrhea (which reduce absorption of nutrients), excessive consumption of alcohol (which promotes hypoglycemia), unusually intense exercise (which promotes cellular glucose uptake and metabolism), and childbirth (which reduces insulin requirements).
Rapid treatment of hypoglycemia is mandatory: if hypoglycemia is allowed to persist, irreversible brain damage or even death may result. In conscious patients, glucose levels can be restored with a fast-acting oral sugar (e.g., glucose tablets, orange juice, sugar cubes, honey, corn syrup, nondiet soda). However, if the swallowing reflex or the gag reflex is suppressed, nothing should be administered by mouth. In cases of severe hypoglycemia, intravenous (IV) glucose is the preferred treatment. Parenteral glucagon is an alternative treatment. (The pharmacology of glucagon is discussed at the end of the chapter.)
In anticipation of hypoglycemic episodes, people with diabetes should always have an oral carbohydrate available (e.g., sugared candy, sugar cubes, glucose tablets). Some prescribers recommend that patients keep glucagon on hand, too—particularly people on insulin therapy. Patients should carry some sort of identification (e.g., a medical alert bracelet) to inform emergency personnel of their condition.
In some patients, hypoglycemia occurs without producing the symptoms noted previously. This is known as hypoglycemia unawareness. As a result, the patient remains unaware of hypoglycemia until blood sugar has become dangerously low. Hypoglycemia unawareness is a particular problem among patients practicing tight glucose control. This is because as patients experience more frequent hypoglycemia, they start to have diminished symptoms over time. The risk for dangerous hypoglycemia can be minimized by frequently monitoring blood glucose. Additionally, current recommendations state that treatment goals should be temporarily loosened (such as for several weeks) for people experiencing hypoglycemia unawareness so that they can regain hypoglycemia awareness.
Hypoglycemia can result in coma. The most definitive diagnosis is made by measuring plasma glucose levels: in hypoglycemic coma, glucose levels are very low.
Other Complications
Hypokalemia.
Insulin promotes uptake of potassium by cells. Insulin activates a membrane-bound enzyme—Na+,K+-ATPase—that pumps potassium into cells and pumps sodium out. Hence, in addition to lowering blood levels of glucose, insulin can lower blood levels of potassium. When insulin dosage is proper, effects on potassium are unremarkable. However, if insulin dosage is excessive, clinically significant hypokalemia can result. Effects on the heart are of greatest concern: hypokalemia can reduce contractility and can cause potentially fatal dysrhythmias.
Lipohypertrophy.
Lipohypertrophy (accumulation of subcutaneous fat) can occur when insulin is injected too frequently at the same site. Fat accumulates because insulin stimulates fat synthesis. When use of the site is discontinued, excess fat is eventually lost. Lipohypertrophy can be minimized through systematic rotation of injection sites.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree