Development of RBCs requires the cooperative interaction of several factors: bone marrow must be healthy; erythropoietin (a stimulant of RBC maturation) must be present; iron must be available for hemoglobin synthesis; and other factors, including vitamin B12 and folic acid, must be available to support synthesis of DNA. If any of these is absent or amiss, anemia will result.
Iron Deficiency
Iron deficiency is the most common nutritional deficiency and the most common cause of nutrition-related anemia. Worldwide, people with iron deficiency number in the hundreds of millions. In the United States about 5% of the population is iron deficient.
Biochemistry and Physiology of Iron
To understand the consequences of iron deficiency as well as the rationale behind iron therapy, we must first understand the biochemistry and physiology of iron. This information is reviewed next.
Metabolic Functions
Iron is essential to the function of hemoglobin, myoglobin (the oxygen-storing molecule of muscle), and a variety of iron-containing enzymes. Most (70%–80%) of the body’s iron is present in hemoglobin. A much smaller amount (10%) is present in myoglobin and iron-containing enzymes.
Fate in the Body
The major pathways for iron movement and utilization are shown in Fig. 45.2. In the discussion that follows, the numbers in parentheses refer to the circled numbers in the figure.
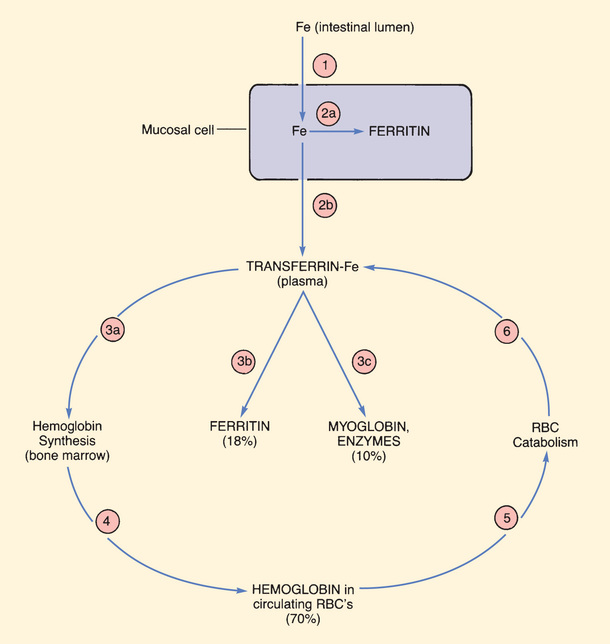
Uptake and Distribution
The life cycle of iron begins with (1) uptake of iron into mucosal cells of the small intestine. These cells absorb 5% to 20% of dietary iron. Their maximal absorptive capacity is 3 to 4 mg/day. Iron in the ferrous form (Fe++) is absorbed more readily than iron in the ferric form (Fe+++). Vitamin C enhances absorption, and food reduces absorption.
After uptake, iron can either (2a) undergo storage within mucosal cells in the form of ferritin (a complex consisting of iron plus a protein used to store iron) or (2b) undergo binding to transferrin (the iron transport protein) for distribution throughout the body.
Utilization and Storage
Iron that is bound to transferrin can undergo one of three fates. Most transferrin-bound iron (3a) is taken up by cells of the bone marrow for incorporation into hemoglobin. Small amounts (3b) are taken up by the liver and other tissues for storage as ferritin. Lastly (3c), some of the iron in plasma is taken up by muscle (for production of myoglobin) and some is taken up by all other tissues (for production of iron-containing enzymes).
Recycling
As Fig. 45.2 depicts, iron associated with hemoglobin undergoes continuous recycling. After hemoglobin is made in bone marrow, iron reenters the circulation (4) as a component of hemoglobin in erythrocytes. (The iron in circulating erythrocytes accounts for about 70% of total body iron.) After 120 days of useful life, RBCs are catabolized (5). Iron released by this process reenters the plasma bound to transferrin (6), and then the cycle begins anew.
Elimination
Excretion of iron is minimal. Under normal circumstances, only 1 mg of iron is excreted each day. At this rate, if none of the lost iron were replaced, body stores would decline by only 10% a year.
Iron leaves the body by several routes. Most excretion occurs through the bowel. Iron in ferritin is lost as mucosal cells slough off, and iron also enters the bowel in bile. Small amounts are excreted in urine and sweat.
Note that, although very little iron leaves the body as a result of excretion (i.e., normal physiologic loss), substantial amounts can leave because of blood loss. Hence menorrhagia, hemorrhage, and blood donations can all cause iron deficiency.
Regulation of Body Iron Content
The amount of iron in the body is regulated through control of intestinal absorption. As noted, most of the iron that enters the body stays in the body. If all dietary iron were readily absorbed, body iron content would rapidly accumulate to a toxic level. However, excessive buildup is prevented through control of iron uptake: as body stores rise, uptake of iron declines; conversely, as body stores become depleted, uptake increases. For example, when body stores of iron are high, only 2% to 3% of dietary iron is absorbed. In contrast, when body stores are depleted, as much as 20% may be absorbed.
Daily Requirements
Requirements for iron are determined largely by the rate of erythrocyte production. When RBC production is low, iron needs are low too. Conversely, when RBC production is high, iron needs rise. Accordingly, among infants and children—individuals whose rapid growth rate requires massive RBC synthesis—iron requirements are high (relative to body weight). In contrast, the daily iron needs of adults are relatively low. Adult men need only 8 mg of dietary iron each day. Adult women need considerably more (15–18 mg/day) to replace iron lost through menstruation.
During pregnancy, requirements for iron increase dramatically, owing to (1) expansion of maternal blood volume and (2) production of RBCs by the fetus. In most cases, the iron needs of pregnant women are too great to be met by diet alone. Consequently, iron supplements (about 27 mg/day) are recommended during pregnancy and for 2 to 3 months after delivery.
Table 45.1 shows the recommended dietary allowances (RDAs) of iron as a function of age. The RDA values in the table are about 10 times greater than actual physiologic need because, on average, only 10% of dietary iron is absorbed. Therefore, if physiologic requirements are to be met, the diet must contain 10 times more iron than we need.
TABLE 45.1
Recommended Dietary Allowances (RDAs) for Iron
| Life Stage | Age | RDA for Iron (mg/day) |
| Infants | 7–12 mo | 11 |
| Children | 1–3 yr | 7 |
| 4–8 yr | 10 | |
| Males | 9–13 yr | 8 |
| 14–18 yr | 11 | |
| ≥19 yr | 8 | |
| Females: nonpregnant, nonlactating | 9–13 yr | 8 |
| 14–18 yr | 15 | |
| 19–50 yr | 18 | |
| ≥51 yr | 8 | |
| Females: pregnant | 14–50 yr | 27* |
| Females: lactating | 14–18 yr | 10 |
| 19–50 yr | 9 |
Dietary Sources
Iron is available in foods of plant and animal origin. Foods especially rich in iron include egg yolk, brewer’s yeast, and wheat germ. Other foods with a high iron content include muscle meats, fish, fowl, cereal grains, beans, and green leafy vegetables. Foods that do not provide much iron include milk and most nongreen vegetables. Because iron can be extracted from cooking utensils, using iron pots and pans can augment dietary iron. Except for individuals who have very high iron requirements (infants, pregnant patients, those undergoing chronic blood loss), the average diet is sufficient to meet iron needs.
Iron Deficiency: Causes, Consequences, and Diagnosis
Causes
Iron deficiency results when there is an imbalance between iron uptake and iron demand. As a rule, the imbalance results from increased demand—not from reduced uptake. The most common causes of increased iron demand are (1) blood volume expansion during pregnancy coupled with RBC synthesis by the growing fetus; (2) blood volume expansion during infancy and early childhood; and (3) chronic blood loss, usually of gastrointestinal (GI) or uterine origin. Rarely, iron deficiency results from reduced iron uptake; potential causes include gastrectomy and sprue.
Consequences
Iron deficiency has multiple effects, the most conspicuous being iron deficiency anemia. In the absence of iron for hemoglobin synthesis, red blood cells become microcytic and hypochromic. The reduced oxygen-carrying capacity of blood results in listlessness, fatigue, and pallor of the skin and mucous membranes. If tissue oxygenation is severely compromised, tachycardia, dyspnea, and angina may result. In addition to causing anemia, iron deficiency impairs myoglobin production and synthesis of iron-containing enzymes. In young children, iron deficiency can cause developmental problems, and in school-aged children, iron deficiency may impair cognition.
Diagnosis
The hallmarks of iron deficiency anemia are (1) the presence of microcytic, hypochromic erythrocytes and (2) the absence of hemosiderin (aggregated ferritin) in bone marrow (Table 45.2). Additional laboratory data that can help confirm a diagnosis include reduced RBC count, reduced reticulocyte hemoglobin content, reduced hemoglobin and hematocrit values, reduced serum iron content, and increased serum iron-binding capacity (IBC).1
TABLE 45.2
Diagnosing Anemia
| Red Cell Size | Classification | Laboratory Values | Common Causes | |
| Microcytic anemia | Microcytic <80 fL | Iron deficiency anemia | ||
| Megaloblastic Anemia | Macrocytic >100 fL | Vitamin B12 deficiency | Vitamin B12 < 200 pg/mL | |
| Folate deficiency | Folate <2 ng/mL | Low dietary intake |
When a diagnosis of iron deficiency anemia is made, it is imperative that the underlying cause be determined. This is especially true when the suspected cause is GI-related blood loss because GI blood loss may be indicative of peptic ulcer disease or GI cancer, conditions that demand immediate treatment.
Oral Iron Preparations
As shown in Table 45.3, iron for oral therapy is available in multiple forms. Of these, the ferrous salts (especially ferrous sulfate) and carbonyl iron are used most often. Accordingly, the following discussion is limited to these iron preparations.
TABLE 45.3
Iron Preparations Available for Oral Therapy
| Iron Preparation | Trade Names | Description |
| Ferrous iron salts: | ||
| Ferrous sulfate | Feosol, FeroSul, Ferodan  , Slow FE, others , Slow FE, others | All four compounds are salts of the ferrous form of iron |
| Ferrous gluconate | Fergon, Floradix, others | |
| Ferrous fumarate | Ferro-Sequels, Hemocyte, Palafer  , others , others | |
| Ferrous aspartate | FE Aspartate | |
| Ferrous bisglycinate | Ferrochel, others | An iron–amino acid chelate |
| Ferric ammonium citrate | Iron Citrate | A ferric iron salt |
| Carbonyl iron | Feosol, Ircon, Icar, others | Microparticles of elemental iron |
| Heme-iron polypeptide | Proferrin | Hemoglobin extracted from porcine RBCs |
| Polysaccharide iron complex | Niferex-150 Forte, Ferrex 150, Triferexx 150  , others , others | Ferric iron complexed to hydrolyzed starch |
Ferrous Iron Salts
We have two basic types of iron salts: ferrous salts and ferric salts. Discussion here is limited to the ferrous iron salts because they are absorbed 3 times more readily than the ferric salts and thus are more widely used. Four ferrous iron salts are available: ferrous sulfate, ferrous gluconate, ferrous fumarate, and ferrous aspartate. All four are equally effective, and with all four, GI disturbances are the major adverse effect.
Ferrous Sulfate
Indications.
Ferrous sulfate is the treatment of choice for iron deficiency anemia. It is also the preferred drug for preventing deficiency when iron needs cannot be met by diet alone (e.g., during pregnancy or chronic blood loss). Ferrous sulfate costs less than ferrous gluconate or ferrous fumarate but has equal efficacy and tolerability.
Adverse Effects
The most significant adverse effects involve the GI tract. These effects, which are dose dependent, include nausea, heartburn, bloating, constipation, and diarrhea. Gastrointestinal reactions are most intense during initial therapy and become less disturbing with continued drug use. Because of their GI effects, oral iron preparations can aggravate peptic ulcers, regional enteritis, and ulcerative colitis. Accordingly, patients with these disorders should not take iron by mouth. In addition to its other GI effects, oral iron may impart a dark green or black color to stools. This effect is harmless and should not be interpreted as a sign of GI bleeding.
Toxicity.
Iron in large amounts is toxic. Poisoning is almost always the result of accidental or intentional overdose, not from therapeutic doses. Death from iron ingestion is rare in adults. By contrast, in young children, iron-containing products remain one of the leading causes of poisoning fatalities. For children, the lethal dose of elemental iron is 2 to 10 g. To reduce the risk for pediatric poisoning, iron should be stored in childproof containers and kept out of reach.
Symptoms.
The effects of iron poisoning are complex. Early reactions include nausea, vomiting, diarrhea, and shock. These are followed by acidosis, gastric necrosis, hepatic failure, pulmonary edema, and vasomotor collapse.
Diagnosis and Treatment.
With rapid diagnosis and treatment, mortality from iron poisoning is low (about 1%). Serum iron should be measured and the intestine x-rayed to determine whether unabsorbed tablets are present. Supportive care is the mainstay of treatment. Gastric lavage may be considered in patients with intentional ingestion of large amounts of iron.
If the plasma level of iron is high (above 500 mcg/dL), it should be lowered with parenteral deferoxamine [Desferal]. Deferoxamine adsorbs iron and thereby prevents toxic effects.
Drug Interactions.
Interaction of iron with other drugs can alter the absorption of iron, the other drug, or both. Antacids reduce the absorption of iron. Coadministration of iron with tetracyclines decreases absorption of both. Ascorbic acid (vitamin C) promotes iron absorption but also increases its adverse effects. Accordingly, attempts to enhance iron uptake by combining iron with ascorbic acid offer no advantage over a simple increase in iron dosage.
Preparations.
Ferrous sulfate is available in standard tablets and in enteric-coated and sustained-release formulations. The enteric-coated and sustained-release products are designed to reduce gastric disturbances. Unfortunately, although side effects may be lowered, these special formulations have disadvantages. First, iron may be released at variable rates, causing variable and unpredictable absorption. Second, these preparations are expensive. Standard tablets do not share these drawbacks.
Some iron products are formulated with vitamin C. The goal is to improve absorption. Unfortunately, the amount in most products is too low to help: more than 200 mg of vitamin C is needed to enhance the absorption of 30 mg of elemental iron.
Trade names for ferrous sulfate products include Feosol, FeroSul, Slow FE, and Ferodan  .
.
Dosage and Administration
Dosing with oral iron can be complicated in that oral iron salts differ with regard to percentage of elemental iron (Table 45.4). Ferrous sulfate, for example, contains 20% iron by weight. In contrast, ferrous gluconate contains only 11.6% iron by weight. Consequently, to provide equivalent amounts of elemental iron, we must use different doses of these iron salts. For example, if we want to provide 100 mg of elemental iron using ferrous sulfate, we need to administer a 500-mg dose. To provide this same amount of elemental iron using ferrous fumarate, the dose would be only 300 mg. In the following discussion, dosage values refer to milligrams of elemental iron, and not to milligrams of any particular iron compound needed to provide that amount of elemental iron.
TABLE 45.4
Commonly Used Oral Iron Preparations
| Iron Preparation | Elemental Iron (% by Weight) | Dose Providing 100 mg Elemental Iron |
| FERROUS IRON SALTS | ||
| Ferrous sulfate | 20 | 500 mg |
| Ferrous sulfate (dried) | 30 | 330 mg |
| Ferrous fumarate | 33 | 300 mg |
| Ferrous gluconate | 11.6 | 860 mg |
| Ferrous aspartate | 16 | 625 mg |
| ELEMENTAL IRON | ||
| Carbonyl iron | 100 | 100 mg |
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


