Chapter 56 The following is a very brief discussion of breast cancer. Much research has been related to risk factors, and new ones have been discovered. Box 56-1 lists the well-established risk factors. A breast cancer risk calculator can be found at http://www.cancer.gov/bcrisktool. It also can be obtained by calling 800-4-CANCER, or through AstraZeneca Pharmaceuticals, which manufactures tamoxifen. Tamoxifen is a nonsteroidal selective estrogen receptor modulator (SERM; see Chapter 39, Osteoporosis Treatment). It competes with estradiol at binding sites in the cell nucleus in breast tissue, altering gene transcription and protein synthesis. This inhibits the growth of estrogen-dependent tumor cells. Tamoxifen acts as an estrogen agonist and has a favorable effect on plasma lipid levels and bone mineral density. However, it may be linked to endometrial malignancy and thromboembolism. Multiple guidelines are available. See the National Guideline Clearinghouse at www.guideline.gov, or the National Comprehensive Cancer Network Clinical Practice Guidelines at www.NCCN.org. (Recommendations may vary depending on whether the patient is premenopausal or postmenopausal.) • Radiotherapy (reduced recurrence) • Tamoxifen plus radiotherapy (reduced recurrence in women with estrogen receptor–positive tumors) • Adjuvant aromatase inhibitors • Adjuvant combination chemotherapy (better than no chemotherapy) • Adjuvant tamoxifen (in women with estrogen receptor–positive tumors) • Anthracycline regimens as adjuvant chemotherapy (better than standard CMF [cyclophosphamide, methotrexate, and fluorouracil] regimens) • Chemotherapy plus monoclonal antibody (trastuzumab) in women with overexpressed HER2/neu oncogene • Combined chemotherapy plus tamoxifen • Less extensive mastectomy (similar survival to more extensive surgery and better cosmetic outcome) • Ovarian ablation in premenopausal women • Radiotherapy after breast-conserving surgery (reduced local recurrence and breast cancer mortality compared with breast-conserving surgery alone) • Radiotherapy after mastectomy in women at high risk of local recurrence • Women diagnosed with early-stage breast cancer who took supplements of vitamin C (ascorbic acid) or vitamin E at least 6 days a week had a lower risk of cancer recurrence with vitamin C, but the differences in breast cancer outcomes were about the same for vitamin E. • The bone-building drug denosumab (Prolia) is FDA approved to help reduce fractures in prostate and breast cancer patients using aromatase inhibitors. • Hormonal treatment with antiestrogens (tamoxifen) or progestins (no significant difference in survival compared with non-taxane combination chemotherapy, so may be preferable in women with estrogen receptor–positive disease) • Selective aromatase inhibitors in postmenopausal women (at least as effective as tamoxifen in delaying disease progression) Tamoxifen was approved in 1994. The other drugs in this chapter are relatively newer. Tamoxifen is used for the prevention of breast cancer in women who are at increased risk (Box 56-1). The benefits are weighed against the risks of drug use (see breast cancer risk calculator at http://www.cancer.gov/bcrisktool). It has a “black box” warning because of the risk of increased endometrial cancer, stroke, pulmonary embolism, and deep venous thrombosis. As with all drugs, the benefits should be weighed against the risks of drug use. Soltamox is a liquid formulation of tamoxifen that has been developed to help improve compliance with medications when the effects of radiation, surgery, or chemotherapy has made it difficult for the patient to swallow the pill form of tamoxifen. Additionally, this formulation is helpful for some people who prefer liquid medications rather than pills or just want to reduce the number of pills they have to take daily. Clinicians may have patients fill out a simple 10-question survey to help assess if they have difficulty swallowing. The EAT-10 is a clinically validated tool available at www.swallowingdifficulties.org and provides results helpful in stimulating discussions about a swallowing problem.
Drugs for Breast Cancer
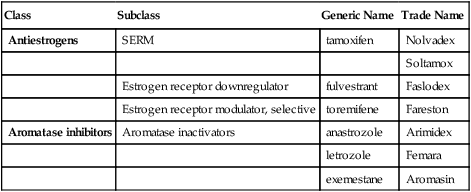
Class
Subclass
Generic Name
Trade Name
Antiestrogens
SERM
tamoxifen
Nolvadex
Soltamox
Estrogen receptor downregulator
fulvestrant
Faslodex
Estrogen receptor modulator, selective
toremifene
Fareston
Aromatase inhibitors
Aromatase inactivators
anastrozole
Arimidex
letrozole
Femara
exemestane
Aromasin
Therapeutic Overview
Assessment
Risk Factors
Mechanism of Action
Antiestrogens
Treatment Principles
Standardized Guidelines
Evidence-Based Recommendations
Local Breast Cancer—Nonmetastatic
Breast Cancer—Metastatic
Pharmacotherapeutic Treatment
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


