Chapter 17 Drugs Affecting Uterine Motility
| Abbreviations | |
|---|---|
| COMT | Catechol-O-methyl transferase |
| COX | Cyclooxygenase |
| IM | Intramuscular |
| IV | Intravenous |
| MLC | Myosin light-chain |
| MLCK | Myosin light-chain kinase |
| MLCP | Myosin light-chain phosphatase |
| NO | Nitric oxide |
| NSAID | Nonsteroidal antiinflammatory drug |
| OT | Oxytocin |
| PG | Prostaglandin |
Therapeutic Overview
There are three clinical uses for uterine stimulants:
during pregnancy can significantly alter uterine responses. This may occur through alterations in receptor density, coupling to effector mechanisms, or other processes.
In general there are four groups of compounds used clinically to stimulate uterine motility. The most potent and specific is oxytocin (OT), which is commonly used to induce or augment labor in late gestation. It is much less useful in early gestation, however, because the uterus responds poorly to OT. The second group consists of the prostaglandins (PGs) of the E or F families. Because the uterus is always responsive to PGs, they can stimulate contractions at any stage of gestation (see Chapter 15). The PGs are used in combination with mifepristone to induce early abortion. They are also commonly used in late gestation and can ripen the cervix and cause myometrial contraction. The third group is the ergot alkaloids (see Chapter 36). These compounds cause intense tonic myometrial contractions, which are undesirable for stimulating labor but are useful for treating postpartum hemorrhage. They are, however, rapidly being replaced by analogs of OT or PGs. The final group is the progesterone receptor antagonists, of which mifepristone is the most widely used (see Chapter 40). These are particularly useful for termination of early pregnancy, when uterine quiescence is dependent principally on progesterone. They have also recently been used to induce labor in late gestation.
There are four clinical uses for uterine relaxants:
Because there is no evidence clearly supporting the superiority of any one tocolytic, their use varies markedly. Magnesium sulfate is used most frequently as a tocolytic agent despite the lack of evidence of effectiveness from well-designed trials. Adrenergic β2 receptor agonists (see Chapter 11), usually ritodrine or terbutaline, have often been prescribed, but their use is declining because of maternal side effects. The nonsteroidal antiinflammatory drugs (NSAIDs) and PG synthesis inhibitors (see Chapter 36) have also been used, although there are concerns about potential adverse effects on the fetus. Similarly, calcium channel blockers (principally nifedipine) are used increasingly, but their efficacy has not been proven. The more recently developed OT antagonist, atosiban, has demonstrated efficacy but may be associated with fetal adverse effects and has not been approved for use as a tocolytic in the United States. Limited research supports the use of nitroglycerin as a nitric oxide (NO) donor to enhance uterine quiescence, and there has been a resurgence of interest in the use of progesterone supplementation in early pregnancy to prevent preterm labor in women at high risk.
Dysmenorrhea is caused by uterine spasms secondary to the release of PGs at the time of endometrial breakdown associated with menstruation. Several NSAIDs relieve the discomfort associated with uterine cramps around the time of menstruation (see Chapter 36).
Mechanisms of Action
| Therapeutic Overview | |
|---|---|
| Uterine Stimulation | Uterine Relaxation |
| Pregnancy termination | Arrest of preterm labor |
| Cervical ripening | Facilitation of intrauterine manipulation |
| Induction of labor | Reversal of pharmacological uterine hyperstimulation |
| Augmentation of labor | Relief of dysmenorrhea |
| Postpartum uterine atony | |
pharmacological characteristics change constantly in response to changes in estrogen and progesterone throughout the menstrual cycle and more so during pregnancy. Most unique are the massive anatomical and physiological changes that transform it during pregnancy. Not surprisingly, the factors regulating uterine contractility, and the effectiveness of drug therapy, change remarkably during the menstrual cycle, pregnancy, and particularly around parturition.
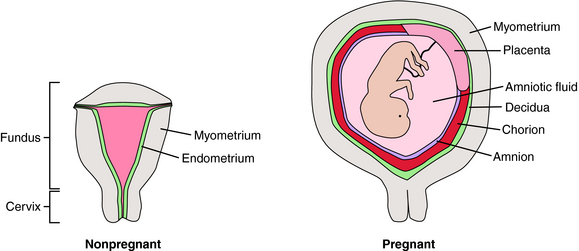
At first glance, the uterus appears anatomically simple (Fig. 17-1). There is a body (fundus) and an outflow tract (cervix) through which the fetus and placenta must pass during parturition. The fundus is composed principally of smooth muscle (myometrium) surrounding the uterine cavity, which is lined with a specialized endometrium containing stromal cells and glandular epithelium. During pregnancy the myometrium undergoes massive hypertrophy and hyperplasia, predominantly under the influence of estrogen. The endometrium is also a target for estrogen and progesterone, changing dramatically throughout the menstrual cycle. In pregnancy, stromal cells enlarge, whereas glandular epithelial cells become less prominent. The pregnant endometrium is termed the decidua. As pregnancy progresses, the fetus grows in a gestational sac composed of two types of fetal tissue—the inner amnion, a single layer of cuboidal epithelial cells with a loose connective tissue matrix, and the outer chorion, which is a continuation of the placental trophoblast that extends from the edge of the placenta and surrounds the entire developing conceptus. As the fetus grows, the amniochorial layer becomes fused with the maternal decidua. Near the time of parturition, the decidua is invaded by cells of the immune system. The timing of parturition is a complex and coordinated event involving fetal tissues as well as the maternal decidua, myometrium, and immune system. It appears that there are several redundant pathways for initiation of labor.
It is important not to view labor simply as the onset of myometrial contractions. For successful parturition, the cervix must also undergo dramatic changes, called ripening. In this process collagen and glycosaminoglycans of the cervix are broken down, and the content of H2O and hyaluronic acid increases, probably as a consequence of the action of matrix metalloproteinases. As a result, the cervix is transformed from a rigid structure that keeps the products of conception confined to the uterus into a soft and pliable structure. During ripening the cervix becomes thin (effacement) and then begins to open (dilation). Active labor contractions ensue to continue the process of dilating the cervix and pushing the fetus through the maternal pelvis. These processes must be well coordinated to ensure normal progressive labor.
Regulation of Myometrial Contractility
Although the processes regulating the myometrium and other smooth muscles are similar in some respects (see Chapters 9, 19 and 24), there are unique aspects in the control of the myometrium that determine its responsiveness to drugs. Much of our understanding of this process is derived from animal models, particularly sheep. In this species the signal for parturition is mediated through the fetus. In the days preceding the onset of labor, the fetal adrenal increases the secretion of cortisol; the resulting increase in fetal serum cortisol induces the synthesis of placental 17-hydroxylase, which catalyzes conversion of placental progesterone to estrogen. The consequential large increase in the maternal serum estrogen/progesterone ratio stimulates increased uterine contractility and labor onset. In most species this “progesterone withdrawal” is thought to be a critical step in transformation of the uterus from its quiescent state during pregnancy into an active state during parturition.
The actions of estrogen and progesterone on the uterus are not well understood. As in other tissues (see Chapter 40), expression of some uterine genes may be increased, whereas others are decreased through interactions with nuclear receptors. The reproductive effects of estrogen appear to use estrogen receptor α, whereas effects of progesterone are mediated primarily through the progesterone receptor B isoform (see Chapter 40). In human pregnancy it has been speculated that a progesterone withdrawal may be caused by increased expression of the progesterone receptor A isoform, which may counteract the effects of progesterone receptor B activation.
The molecular mechanism that underlies myometrial contraction is similar in most respects to that of other smooth muscle (see Chapter 24). The final focal point of the contractile response is the interaction of phosphorylated myosin light chains (MLCs) with actin. Phosphorylation of MLCs is regulated by the balance of activity between MLC kinase (MLCK) and MLC phosphatase (MLCP
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree