Calcium in Blood
The normal value for total serum calcium is 10 mg/dL (2.5 mmol/L, 5 mEq/L). Of this total, about 50% is bound to proteins and other substances and hence is unavailable for use. The remaining 50% is present as free, ionized calcium—the form that participates in physiologic processes.
Absorption and Excretion
Absorption
Absorption of calcium takes place in the small intestine. Under normal conditions, about one third of ingested calcium is absorbed. Absorption is increased by parathyroid hormone (PTH) and vitamin D. In contrast, glucocorticoids decrease calcium absorption. Also, foods with insoluble fiber and phytic acid, such as whole-grain cereals and wheat bran, and foods containing oxalates, such as spinach, can interfere with calcium absorption.
Excretion
Calcium excretion is primarily renal. The amount lost is determined by glomerular filtration and the degree of tubular reabsorption. Excretion can be reduced by PTH, vitamin D, and thiazide diuretics (e.g., hydrochlorothiazide). Conversely, excretion can be increased by loop diuretics (e.g., furosemide), calcitonin, and loading with sodium. In addition to calcium lost in urine, substantial amounts can be lost in breast milk.
Physiologic Regulation of Calcium Levels
Blood levels of calcium are tightly controlled. Three processes are involved:
Regulation of these processes is under the control of three factors: parathyroid hormone, vitamin D, and calcitonin, as shown in Table 59.2. Note that preservation of calcium levels in blood takes priority over preservation of calcium in bone. Therefore, if serum calcium is low, calcium will be resorbed from bone and transferred to the blood—even if resorption compromises the structural integrity of bone.
TABLE 59.2
Effects of Parathyroid Hormone, Vitamin D, and Calcitonin on Calcium and Phosphate
| PTH | Vitamin D | Calcitonin | |
| CALCIUM | |||
| Plasma calcium level | Increase | Increase | Decrease |
| Intestinal calcium absorption | Increase | Increase | No effect |
| Renal calcium excretion | Decrease | Decrease | Increase |
| Calcium resorption from bone | Increase | Increase | Decrease |
| PHOSPHATE | |||
| Plasma phosphate level | Decrease | Increase | |
Parathyroid Hormone
Release of PTH is regulated primarily by calcium, acting through calcium-sensing receptors on cells of the parathyroid gland. When calcium levels are high, activation of the calcium-sensing receptors is increased, causing secretion of PTH to be suppressed. Conversely, when calcium levels are low, receptor activation is reduced, causing PTH release to rise. PTH then restores calcium to normal levels by three mechanisms:
• PTH promotes calcium resorption from bone.
• PTH promotes tubular reabsorption of calcium that had been filtered by the kidney glomerulus.
• PTH promotes activation of vitamin D and thereby promotes increased absorption of calcium from the intestine.
In addition to its effects on calcium, PTH reduces plasma levels of phosphate.
Vitamin D
Vitamin D is similar to PTH in that both agents increase plasma calcium levels, and they do so by the same mechanisms: (1) increasing calcium resorption from bone, (2) decreasing calcium excretion by the kidney, and (3) increasing calcium absorption from the intestine. Vitamin D differs from PTH in that vitamin D elevates plasma levels of phosphate, whereas PTH reduces levels of phosphate.
Calcitonin
Calcitonin, a hormone produced by the thyroid gland, decreases plasma levels of calcium. Hence, calcitonin acts in opposition to PTH and vitamin D. Calcitonin is released from the thyroid gland when calcium levels in blood rise too high. Calcitonin lowers calcium levels by inhibiting the resorption of calcium from bone and increasing calcium excretion by the kidney. Unlike PTH and vitamin D, calcitonin does not influence calcium absorption.
Calcium-Related Pathophysiology
Hypercalcemia
Clinical Presentation
Hypercalcemia is usually asymptomatic. When symptoms are present, they often involve the kidney (damage to tubules and collecting ducts, resulting in polyuria, nocturia, and polydipsia), gastrointestinal (GI) tract (nausea, vomiting, and constipation), and central nervous system (lethargy and depression). Hypercalcemia may also result in dysrhythmias and deposition of calcium in soft tissues. As noted previously, consuming too much supplemental calcium increases the risk for vascular calcification, myocardial infarction, stroke, and kidney stones.
Causes
Hypercalcemia may arise from a variety of causes. Life-threatening elevations in calcium are most often the result of cancer. Hyperparathyroidism is another common cause of severe hypercalcemia. Additional causes include vitamin D intoxication, sarcoidosis, and use of thiazide diuretics.
Treatment
Calcium levels can be lowered with drugs that (1) promote urinary excretion of calcium, (2) decrease mobilization of calcium from bone, (3) decrease intestinal absorption of calcium, and (4) form complexes with free calcium in blood. For severe hypercalcemia, initial therapy consists of replacing lost fluid with intravenous (IV) saline, followed by diuresis using IV saline and a loop diuretic (e.g., furosemide). Other agents for lowering calcium include inorganic phosphates (which promote calcium deposition in bone and reduce calcium absorption); edetate disodium (EDTA, which binds calcium and promotes its excretion); glucocorticoids (which reduce intestinal absorption of calcium); and a group of drugs—calcitonin, bisphosphonates (e.g., pamidronate), and gallium nitrate—that inhibit resorption of calcium from bone. Cinacalcet [Sensipar], a drug that suppresses PTH section, can be used for hypercalcemia associated with hyperparathyroidism.
Hypocalcemia
Clinical Presentation
Hypocalcemia increases neuromuscular excitability. As a result, tetany, convulsions, and spasm of the pharynx and other muscles may occur.
Cause
Hypocalcemia is caused most often by a deficiency of either PTH, vitamin D, or dietary calcium. Other causes include chronic renal failure and long-term use of certain medications, such as magnesium-based laxatives and drugs used to manage osteoporosis (e.g., bisphosphonates and denosumab).
Treatment
Severe hypocalcemia is corrected by infusing an IV calcium preparation, usually calcium gluconate (see Chapter 89). After calcium levels have been restored, an oral calcium salt (e.g., calcium citrate) can be given for maintenance. Vitamin D should be included in the regimen if there is a coexisting deficiency.
Osteomalacia
Osteomalacia results from insufficient vitamin D. In the absence of vitamin D, mineralization of bone is impaired, resulting in bowing of the legs, fractures of the long bones, and kyphosis. In addition, patients may experience diffuse, dull, aching bone pain. Treatment consists of vitamin D replacement therapy.
Osteoporosis
Osteoporosis, the most common disorder of calcium metabolism, is characterized by low bone mass and increased bone fragility. Osteoporosis is discussed at length in a later section.
Paget Disease of Bone
Clinical Presentation
Paget disease of bone is a chronic condition seen most frequently in adults older than 40 years. After osteoporosis, Paget disease is the most common disorder of bone in the United States. The disease is characterized by increased bone resorption and replacement of the resorbed bone with abnormal bone. Increased bone turnover causes elevation in serum alkaline phosphatase (reflecting increased bone deposition) and increased urinary hydroxyproline (reflecting increased bone resorption). It is important to note that alterations in bone homeostasis do not occur evenly throughout the skeleton. Rather, alterations occur locally, most often in the pelvis, femur, spine, skull, and tibia. Although most people with Paget disease are asymptomatic, about 10% experience bone pain and osteoarthritis. Skeletal deformity may also occur. Bone weakness may lead to fractures. Neurologic complications may occur secondary to compression of the spinal cord, spinal nerves, and cranial nerves. If bone associated with hearing is affected, deafness may result.
Treatment
Asymptomatic patients are usually not treated. Mild pain can be managed with analgesics and antiinflammatory agents. When the disease is more severe, a bisphosphonate is the treatment of choice. Benefits derive from suppressing bone resorption.
Hypoparathyroidism
Reductions in PTH usually result from inadvertent removal of the parathyroid glands during surgery on the thyroid gland. Lack of PTH causes hypocalcemia, which in turn may produce paresthesias, tetany, skeletal muscle spasm, laryngospasm, and convulsions. Symptoms can be relieved with calcium supplements and vitamin D.
Hyperparathyroidism
Primary Hyperparathyroidism
Primary hyperparathyroidism usually results from a benign parathyroid adenoma. The resulting increase in PTH secretion causes hypercalcemia and lowers serum phosphate. Hypercalcemia can cause skeletal muscle weakness, constipation (from decreased smooth muscle tone), and central nervous system (CNS) symptoms (lethargy, depression). Hypercalciuria and hyperphosphaturia are also present and may cause renal calculi. Mobilization of calcium and phosphate from bone may produce bone abnormalities. Management of hyperparathyroidism is typically overseen by an endocrinologist. The only definitive treatment for primary hyperparathyroidism is surgical resection of the parathyroid glands. Hypercalcemia can be managed with calcium-lowering drugs, including cinacalcet [Sensipar], a drug that suppresses PTH secretion.
Secondary Hyperparathyroidism
Secondary hyperparathyroidism is a common complication of chronic kidney disease, occurring in nearly all patients undergoing dialysis. The disorder is characterized by high levels of PTH and disturbances of calcium and phosphorus homeostasis. Traditionally, the disorder has been managed with a vitamin D sterol (e.g., paricalcitol) and calcium-containing phosphate-binding agents. However, these treatments frequently make mineral homeostasis worse. Cinacalcet [Sensipar] has been advocated by some experts because of its ability to reduce PTH levels while having a positive effect on calcium and phosphorus; however, others (e.g., the Kidney Disease: Improving Global Outcomes [KDIGO] working group), recommend against its use in the early or predialysis stages of kidney disease.
Drugs for Disorders Involving Calcium and Bone Mineralization
Calcium Salts
Calcium salts are available in oral and parenteral formulations for treating hypocalcemic states. These salts differ in their percentages of elemental calcium, which must be accounted for when determining dosage.
Oral formulations are presented here. Information on parenteral calcium preparations are available in Chapter 89.
Therapeutic Uses
Oral calcium preparations are used to treat mild hypocalcemia. In addition, calcium salts are taken as dietary supplements. As discussed in Chapter 48, calcium supplements may have the added benefit of reducing symptoms of premenstrual syndrome. Also, data indicate that calcium supplements can produce a significant, albeit modest, reduction in recurrence of colorectal adenomas.
Adverse Effects
When calcium is taken chronically in high doses (3–4 g/day), hypercalcemia can result. Hypercalcemia is most likely in patients who are also receiving large doses of vitamin D. Signs and symptoms include GI disturbances (nausea, vomiting, constipation), renal dysfunction (polyuria, nephrolithiasis), and CNS effects (lethargy, depression). In addition, hypercalcemia may cause cardiac dysrhythmias and deposition of calcium in soft tissue. Hypercalcemia can be minimized with frequent monitoring of plasma calcium content.
Drug Interactions
Glucocorticoids (e.g., prednisone) reduce absorption of oral calcium, leading to osteoporosis with long-term use. Calcium reduces absorption of a number of drugs when administered together. These drugs include tetracycline and quinolone antibiotics, thyroid hormone, the anticonvulsant phenytoin, and bisphosphonates. Thiazide diuretics decrease renal calcium excretion and may thereby cause hypercalcemia; however, loop diuretics increase calcium excretion and may cause hypocalcemia.
Food Interactions
Certain foods contain substances that can suppress calcium absorption. One such substance—oxalic acid—is found in spinach, rhubarb, Swiss chard, and beets. Phytic acid, another depressant of calcium absorption, and insoluble fiber, which also hampers absorption, are present in bran and whole-grain cereals. Oral calcium supplements should not be administered with these foods.
Preparations and Dosage
The calcium salts available for oral administration are shown in Table 59.3. Note that the dosage required to provide a particular amount of elemental calcium differs among preparations. Calcium carbonate, for example, has the highest percentage of calcium. Chewable tablets are preferred to standard tablets because of more consistent bioavailability. Bioavailability of calcium citrate appears especially good, owing to high solubility. When calcium supplements are taken, total daily calcium intake (dietary plus supplemental) should equal the values in Table 59.1. To help ensure adequate absorption, no more than 600 mg should be consumed at one time.
TABLE 59.3
Oral Calcium Salts
| Generic Name | Trade Name | Calcium Content | Dose Providing 1000 mg of Calcium |
| Calcium acetate | PhosLo, Calphron, Eliphos | 25% | 4 g |
| Calcium carbonate | Tums, Rolaids, others | 40% | 2.6 g |
| Calcium citrate | Citracal, Cal-Cee | 21% | 4.8 g |
| Calcium glubionate | Calcionate | 6.6% | 15.2 g |
| Calcium gluconate* | Cal-G | 9% | 11 g |
| Calcium lactate | Cal-Lac | 13% | 7.6 g |
| Tricalcium phosphate | Posture | 39% | 2.6 g |
Vitamin D
The term vitamin D refers to two compounds: ergocalciferol (vitamin D2) and cholecalciferol (vitamin D3). Vitamin D3 is the form of vitamin D produced naturally in humans when our skin is exposed to sunlight. Vitamin D2 is a form of vitamin D that occurs in plants. Vitamin D2 is used as a prescription drug and to fortify foods. Both forms are used in over-the-counter supplements. It is important to note that both forms of vitamin D produce nearly identical biologic effects. Therefore, rather than distinguishing between them, we will use the term vitamin D to refer to vitamins D2 and D3 collectively.
Therapeutic Uses
Vitamin D is essential for bone health, owing to its effects on calcium utilization. The primary indications for vitamin D are vitamin D deficiency and associated conditions such as rickets, osteomalacia, and hypoparathyroidism.
Some studies suggest that vitamin D may also protect against diabetes, arthritis, cardiovascular disease, autoimmune disorders, and cancers of the breast, colon, prostate, and ovary. However, according to the IOM report on calcium and vitamin D, the available data are insufficient to support health claims beyond bone health. Until more definitive data are available, the possibility of additional benefits remains open, but not proved.
Physiologic Actions
Vitamin D is an important regulator of calcium and phosphorus homeostasis. Vitamin D increases blood levels of both elements, primarily by increasing their absorption from the intestine and promoting their resorption from bone. In addition, vitamin D reduces renal excretion of calcium and phosphate (although the quantitative significance of this effect is not clear). With usual doses of vitamin D, there is no net loss of calcium from bone. However, vitamin D can promote bone decalcification if serum calcium concentrations cannot be maintained by increasing intestinal calcium absorption.
Sources and Daily Requirements
Sources
Vitamin D is obtained through the diet, supplements, and exposure to sunlight. With the exception of shiitake mushrooms and oily fish (e.g., salmon, tuna), natural foods have very little vitamin D. Accordingly, dietary vitamin D is obtained mainly through vitamin D–fortified foods, especially cereals, milk, yogurt, margarine, cheese, and orange juice.
Requirements
In 2010, the IOM issued revised guidelines for vitamin D intake. They now recommend the following:
• For children younger than 1 year, 400 international units (IU)/day
• For all people aged 1 through 70 years, 600 IU/day
These recommendations are based on the assumption that people get very little of their vitamin D from exposure to sunlight.
According to the IOM report, most people in North America have blood levels of vitamin D in the range needed to support good bone health and hence do not need vitamin D supplements. Whether taking supplements would confer other benefits remains to be proved.
Vitamin D Deficiency
Vitamin D deficiency is defined by a serum concentration of 25-hydroxyvitamin D (25-[OH]D) below 20 ng/mL. (Levels above 20 ng/mL are sufficient to maintain bone health.) In actual practice, the target level of 25-(OH)D is usually 30 to 60 ng/mL.
How much vitamin D is needed to treat deficiency? In 2011, The Endocrine Society made the following recommendations:
Much higher doses are needed for patients who are obese and for those taking glucocorticoids and other drugs that suppress calcium absorption or that increase calcium excretion.
Screening for vitamin D deficiency is recommended for patients at risk, including those who are pregnant, are obese, or have dark skin (because, compared with light-skinned people, they make less vitamin D in response to sunlight). For others, the U.S. Preventive Services Task Force (USPSTF), in their 2014 update, declined to recommend for or against vitamin D deficiency screening for nonpregnant adults 18 years and older.
Activation of Vitamin D
To affect calcium and phosphate metabolism, vitamin D must first undergo activation. The extent of activation is carefully regulated and is determined by calcium availability: when plasma calcium falls, activation of vitamin D is increased. The pathways for activating vitamins D2 and D3 are shown in Fig. 59.2.
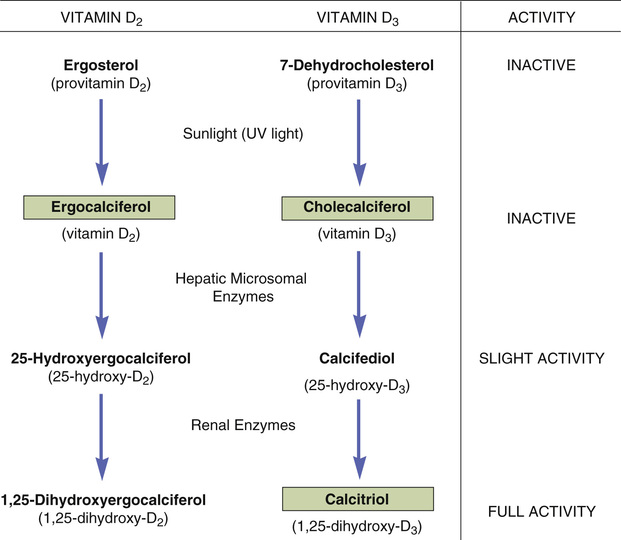
Let’s begin by focusing on vitamin D3, the natural human vitamin. Vitamin D3 (cholecalciferol) is produced in the skin through the action of sunlight on provitamin D3 (7-dehydrocholesterol). Neither provitamin D3 nor vitamin D3 itself possesses significant biologic activity. In the next reaction, enzymes in the liver convert cholecalciferol into calcifediol, which serves as a transport form of vitamin D3 and possesses only slight biologic activity. In the final step, calcifediol is converted into the highly active calcitriol. This reaction occurs in the kidney and can be stimulated by (1) PTH, (2) a drop in dietary vitamin D, and (3) a fall in plasma levels of calcium.
Vitamin D2 is activated by the same enzymes that activate vitamin D3. As we saw with vitamin D3, only the last compound in the series (in this case 1,25-dihydroxyergocalciferol) has significant biologic activity.
Pharmacokinetics
As a rule, vitamin D is administered orally and then absorbed from the small intestine. Bile is essential for absorption. In the absence of sufficient bile, intramuscular (IM) dosing may be required. In the blood, vitamin D is transported complexed with vitamin D–binding protein. Storage of vitamin D occurs primarily in the liver. As discussed, vitamin D undergoes metabolic activation. Reactions that occur in the liver produce the major transport form of vitamin D. A later reaction (in the kidney) produces the fully active form. Excretion of vitamin D is through the bile. Urinary excretion is minimal.
Viewing Vitamin D as a Hormone
Although referred to as a vitamin, vitamin D has all the characteristics of a hormone. With sufficient exposure to sunlight, the body can manufacture all the vitamin D it needs. Hence, under ideal conditions, external sources of vitamin D appear unnecessary. After its production in the skin, vitamin D travels to other locations (liver, kidney) for activation. Like other hormones, activated vitamin D then travels to various sites in the body (bone, intestine, kidney) to exert regulatory actions. Also like other hormones, vitamin D undergoes feedback regulation: as plasma levels of calcium fall, activation of vitamin D increases; when plasma levels of calcium return to normal, activation of vitamin D declines.
Toxicity (Hypervitaminosis D)
Serious vitamin D toxicity (hypervitaminosis D) can be produced by vitamin D doses that exceed 1000 IU/day (in infants) and 50,000 IU/day (in adults). Poisoning occurs most commonly in children; causes include accidental ingestion by the child and excessive dosing with vitamin D by parents. Doses of potentially toxic magnitude are also encountered clinically. When huge therapeutic doses are used, the margin of safety is small, and patients should be monitored closely for signs of poisoning.
Clinical Presentation
Most signs and symptoms of vitamin D toxicity occur secondary to hypercalcemia. Early symptoms include weakness, fatigue, nausea, vomiting, anorexia, abdominal cramping, and constipation. With persistent and more severe hypercalcemia, kidney function is affected, resulting in polyuria, nocturia, and proteinuria, in addition to neurologic symptoms such as seizures, confusion, and ataxia. Cardiac dysrhythmia and coma may occur. Calcium deposition in soft tissues can damage the heart, blood vessels, and lungs; calcium deposition in the kidneys can cause nephrolithiasis. Very large doses of vitamin D can cause decalcification of bone, resulting in osteoporosis; mobilization of bone calcium can occur despite the presence of high calcium concentrations in blood. In children, vitamin D poisoning can suppress growth for 6 months or longer.
Treatment
Treatment consists of stopping vitamin D intake, reducing calcium intake, and increasing fluid intake. Glucocorticoids may be given to suppress calcium absorption. If hypercalcemia is severe, renal excretion of calcium can be accelerated using a combination of IV saline and furosemide.
Preparations, Dosage, and Administration
There are five preparations of vitamin D. Three of these—ergocalciferol, cholecalciferol, and calcitriol—are identical to forms of vitamin D that occur naturally. The other two—paricalcitol and doxercalciferol—are synthetic derivatives of natural vitamin D. (The naturally occurring preparations are highlighted in green boxes in Fig. 59.2.) Individual vitamin D preparations differ in their clinical applications.
Two forms of vitamin D—vitamin D3 (cholecalciferol) and vitamin D2 (ergocalciferol)—are used routinely as dietary supplements. Of the two, vitamin D3 is preferred because it is more effective than vitamin D2 at raising blood levels of 25-(OH)D, the active form of vitamin D in the body.
Vitamin D is almost always administered by mouth. Dosage is usually prescribed in international units (IU). (One IU is equivalent to the biologic activity in 0.025 mcg of vitamin D3.) Daily dosages of vitamin D range from 400 IU (for dietary supplementation) to as high as 500,000 IU (for vitamin D–resistant rickets). Additional information on preparation, dosage, and administration is available in Table 59.4.
TABLE 59.4
Indications, Preparation, and Dosage of Vitamin D Preparations
| Drug Name | Indications | Preparation | Dosage |
| Ergocalciferol (vitamin D2) [Calciferol Drops, Drisdol] | Hypoparathyroidism, vitamin D−resistant rickets, familial hypophosphatemia | ||
| Cholecalciferol (vitamin D3) [Delta-D] | Prophylaxis and treatment of vitamin D deficiency | ||
Calcitriol (1,25-Dihydroxy-D3) [Rocaltrol, Vectical, Calcijex  , Silkis , Silkis  ] ] | Hypoparathyroidism and management of hypocalcemia in patients undergoing chronic renal dialysis | ||
| Doxercalciferol [Hectorol] | Prevention and treatment of secondary hyperparathyroidism in patients undergoing chronic renal dialysis | Capsules: 0.5, 1, and 2.5 mcg* | |
| Paricalcitol [Zemplar] | Prevention and treatment of secondary hyperparathyroidism in patients undergoing chronic renal dialysis | Capsules: 1, 2, and 4 mcg* |
Calcitonin-Salmon
Calcitonin-salmon [Miacalcin, Fortical], a form of calcitonin derived from salmon, is similar in structure to calcitonin synthesized by the human thyroid. Salmon calcitonin produces the same metabolic effects as human calcitonin but has a longer half-life and greater milligram potency. The drug is usually given by nasal spray but can also be given by injection. Intranasal calcitonin was removed from the Canadian market in 2013 because of an increased risk for malignancy associated with this formulation; however, it remains available in the United States.
Actions
Calcitonin has two principal actions: (1) it inhibits the activity of osteoclasts and thereby decreases bone resorption, and (2) it inhibits tubular resorption of calcium and thereby increases calcium excretion. As a result of decreasing bone turnover, calcitonin decreases alkaline phosphatase in blood and increases hydroxyproline in urine. Preparations and dosages for the different indications are presented in Table 59.5.
TABLE 59.5
Indications, Preparation, and Dosage of Calcitonin
| Drug Name | Indications | Preparation | Dosage |
| Intranasal salmon calcitonin [Miacalcin, Fortical] | Management of postmenopausal osteoporosis | Metered dose spray device delivers 200 IU per activation | 200 IU (1 spray) each day, alternating nares daily |
| Parenteral salmon calcitonin [Miacalcin] | Postmenopausal osteoporosis, Paget disease of bone, hypercalcemia | 2-mL vials containing 200 IU/mL Administration is IM or subQ. Dosages are the same for both routes. |
Therapeutic Uses
Osteoporosis
Calcitonin-salmon, given by nasal spray or injection, is indicated for treatment of established postmenopausal osteoporosis—but not for prevention. Benefits derive from suppressing bone resorption. The treatment program should include supplemental calcium and adequate intake of vitamin D.
Paget Disease of Bone
Calcitonin is helpful in moderate to severe Paget disease and is the drug of choice for rapid relief of pain associated with the disorder. Benefits occur secondary to inhibition of osteoclasts. Neurologic symptoms caused by spinal cord compression may be reduced.
Hypercalcemia
Calcitonin can lower plasma calcium levels in patients with hypercalcemia secondary to hyperparathyroidism, vitamin D toxicity, and cancer. Levels of calcium (and phosphorus) are reduced owing to inhibition of bone resorption and increased renal excretion of calcium. Although calcitonin is effective against hypercalcemia, it is not a preferred treatment.
Adverse Effects
With intranasal dosing, nasal dryness and irritation are the most common complaints. As previously mentioned, studies demonstrating an increase in malignancies associated with nasal administration prompted withdrawal of this drug in Canada. After parenteral (IM, subcutaneous [subQ]) administration, about 10% of patients experience nausea, which diminishes with time. An additional 10% have inflammatory reactions at the injection site. Flushing of the face and hands may also occur. When salmon calcitonin is taken for a year or longer, neutralizing antibodies often develop. In some patients, these antibodies bind enough calcitonin to prevent therapeutic effects.
Bisphosphonates
Bisphosphonates are structural analogs of pyrophosphate, a normal constituent of bone. These drugs undergo incorporation into bone, and then inhibit bone resorption by decreasing the activity of osteoclasts. Principal indications are postmenopausal osteoporosis, osteoporosis in men, glucocorticoid-induced osteoporosis (GIOP), Paget disease of bone, and hypercalcemia of malignancy. Bisphosphonates may also help prevent and treat bone metastases in patients with cancer (see Chapter 82).
Bisphosphonates differ with respect to indications, routes, and dosing schedules. Some bisphosphonates are given by oral (PO) route, some by IV route, and some by both routes. Dosing schedules vary from as often as once a day (with oral agents) to as seldom as once every 2 years (with IV zoledronate). As with many drugs for osteoporosis and similar bone disorders, calcium and vitamin D supplements are recommended if there is inadequate dietary intake.
Four bisphosphonates approved for management of osteoporosis and GIOP are currently available in the United States. These are alendronate [Fosamax, Fosamax Plus D, Binosto], ibandronate [Boniva], risedronate [Actonel, Atelvia], and zoledronate [Reclast, Zometa, Aclasta  ]. Additional bisphosphonates have been approved for treatment of Paget disease (e.g., tiludronate [Skelid], etidronate [generic only]) or complications of malignancy (e.g., pamidronate [Aredia]); however, because these conditions are typically managed by endocrinologists, rheumatologists, and oncologists, they have been omitted from this chapter.
]. Additional bisphosphonates have been approved for treatment of Paget disease (e.g., tiludronate [Skelid], etidronate [generic only]) or complications of malignancy (e.g., pamidronate [Aredia]); however, because these conditions are typically managed by endocrinologists, rheumatologists, and oncologists, they have been omitted from this chapter.
Alendronate is the most widely used oral bisphosphonate. It will serve as our prototype for the family.



