Drug Toxicity and Poisoning
Pharmacology intersects with toxicology when the physiological response to a drug is an adverse effect. A poison is any substance, including any drug, that has the capacity to harm a living organism. Poisoning generally implies that damaging physiological effects result from exposure to pharmaceuticals, illicit drugs, or chemicals.
DOSE-RESPONSE
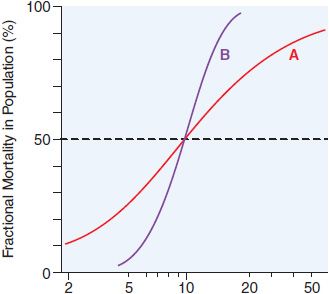
There is a graded dose-response relationship in an individual and a quantal dose-response relationship in the population (see Chapters 2 and 3). Graded doses of a drug given to an individual usually result in a greater magnitude of response as the dose increases. In a quantal dose-response relationship, the percentage of the population affected increases as the dose is raised; the relationship is quantal in that the effect is judged to be either present or absent in a given individual. This quantal dose-response phenomenon is used to determine the median lethal dose (LD50) of drugs, as defined in Figure 4–1.
Figure 4–1 Dose-response relationships. The LD50 of a compound is determined experimentally, usually by administration of the chemical to mice or rats (orally or intraperitoneally). The midpoint of the curve representing percent of population responding (response here is death) versus dose (log scale) represents the LD50, or the dose of drug that is lethal in 50% of the population. The LD50 values for both compounds are the same (~10 mg/kg); however, the slopes of the dose-response curves are quite different. Thus, at a dose equal to one-half the LD50 (5 mg/kg), less than 5% of the animals exposed to compound B would die, but 30% of the animals given compound A would die.
One can also determine a quantal dose-response curve for the therapeutic effect of a drug to generate a median effective dose (ED50), the concentration of drug at which 50% of the population will have the desired response, and a quantal dose-response curve for lethality by the same agent. These 2 curves can be used to generate a therapeutic index (TI), which quantifies the relative safety of a drug:
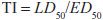
Clearly, the higher the ratio, the safer the drug.
Values of TI vary widely, from 1-2 to >100. Drugs with a low TI must be administered with caution (e.g., cardiac glycoside digitalis and cancer chemotherapeutic agents). Agents with very high TI (e.g., penicillin) are extremely safe in the absence of a known allergic response in a given patient. Note that use of median doses fails to consider that the slopes of the dose-response curves for therapeutic and lethal (toxic) effects may differ (Figure 4–1). As an alternative the ED99 for the therapeutic effect can be compared to the LD1 for lethality (toxic effect), to yield a margin of safety.
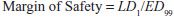
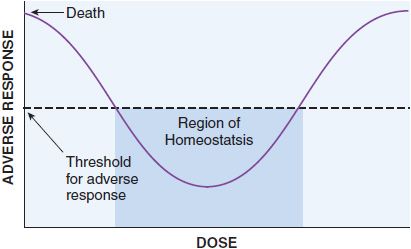
These quantal dose-response relationships are typical sigmoidal dose-response curves. However, not all dose-response curves follow this shape. U-shaped dose-response curves can be observed for essential metals and vitamins (Figure 4–2). At low dose, adverse effects are observed since there is a deficiency of these nutrients to maintain homeostasis. As dose increases, homeostasis is achieved, and the bottom of the U-shaped dose-response curve is reached. As dose increases to surpass the amount required to maintain homeostasis, overdose toxicity can ensue. Thus, adverse effects are seen at both low and high dose.
Figure 4–2 U-Shaped dose-response curve for essential metals and vitamins. Vitamins and essential metals are essential for life and their lack can cause adverse responses (plotted on the vertical axis), as can their excess, giving rise to a U-shaped dose-dependence curve.
PHARMACOKINETICS VERSUS TOXICOKINETICS
Absorption, distribution, metabolism, and elimination (ADME; see Chapters 2, 5, and 6) may differ significantly after poisoning, and these differences can profoundly alter treatment decisions and prognosis. The pharmacokinetics of a drug under circumstances that produce toxicity or excessive exposure are referred to as toxicokinetics. Ingesting larger than therapeutic doses of a pharmaceutical may prolong its absorption, alter its protein binding and apparent volume of distribution, and change its metabolic fate. When confronted with a potential poisoning, 2 questions should be foremost in the clinician’s mind:
• How long will an asymptomatic patient need to be monitored (drug absorption and dynamics)?
• How long will it take an intoxicated patient to get better (drug elimination and dynamics)?
DRUG ABSORPTION. Aspirin poisoning is a leading cause of overdose morbidity and mortality as reported to U.S. poison control centers. In therapeutic dosing, aspirin reaches peak plasma concentrations in ~1 h. However, aspirin overdose may cause spasm of the pyloric valve, delaying entry of the drug into the small intestine. Aspirin, especially enteric-coated forms, may coalesce into bezoars, reducing the effective surface area for absorption. Peak plasma salicylate concentrations from aspirin overdose may not be reached for 4-35 h after ingestion.
DRUG ELIMINATION. After therapeutic dosing, valproic acid has an elimination t½ of ~14 h. Valproic acid poisoning may lead to coma. In predicting the duration of coma, it is important to consider that, after overdose, first-order metabolic processes appear to become saturated and the apparent elimination t½ may exceed 30-45 h. Table 4–1 lists some pharmaceuticals notorious for their predilection to have initial symptoms develop after a typical 4-6 h emergency medical observation period.
Table 4–1
Drugs That Commonly Manifest Initial Symptoms More Than 4-6 Hours after Oral Overdosea
TYPES OF THERAPEUTIC DRUG TOXICITY
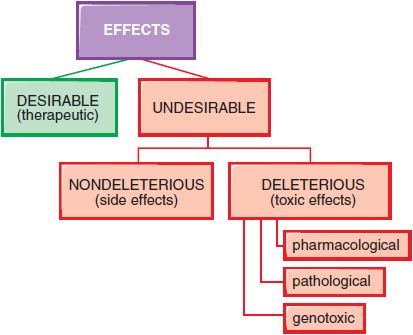
In therapeutics, a drug typically produces numerous effects, but usually only 1 is sought as the primary goal of treatment; most of the other effects are undesirable effects for that therapeutic indication (Figure 4–3). Side effects of drugs usually are bothersome but not deleterious. Other undesirable effects may be characterized as toxic effects.
Figure 4–3 Spectrum of the effects of pharmaceuticals.
DOSE-DEPENDENT REACTIONS. Toxic effects of drugs may be classified as pharmacological, pathological, or genotoxic. Typically, the incidence and seriousness of the toxicity is proportionately related to the concentration of the drug in the body and to the duration of the exposure.
Pharmacological Toxicity. The CNS depression produced by barbiturates is largely predictable in a dose-dependent fashion. The progression of clinical effects goes from anxiolysis to sedation to somnolence to coma. Similarly, the degree of hypotension produced by nifedipine is related to the dose of the drug administered. Tardive dyskinesia (see Chapter 16), an extrapyramidal motor disorder associated with use of antipsychotic medications, seems to be dependent on duration of exposure. Pharmacological toxicity can also occur when the correct dose is given: there is phototoxicity associated with exposure to sunlight in patients treated with tetracyclines, sulfonamides, chlorpromazine, and nalidixic acid.
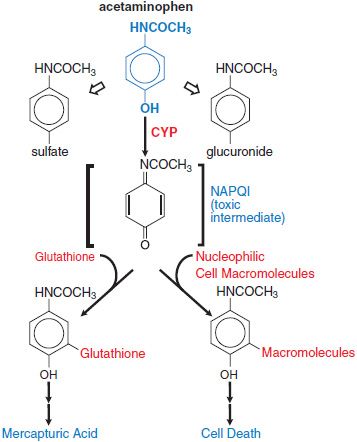
Pathological Toxicity. Acetaminophen is metabolized to nontoxic glucuronide and sulfate conjugates, and to a highly reactive metabolite N-acetyl-p-benzoquinoneimine (NAPQI) via CYP isoforms. At therapeutic dose, NAPQI binds to nucleophilic glutathione; but, in overdose, glutathione depletion may lead to the pathological finding of hepatic necrosis (Figure 4–4).
Figure 4–4 Pathways of acetaminophen metabolism and toxicity. The toxic intermediate NAPQI is N-acetyl-p-benzoquinoneimine.
Genotoxic Effects. Ionizing radiation and many environmental chemicals are known to injure DNA, and may lead to mutagenic or carcinogenic toxicities. Many of the cancer chemotherapeutic agents (see Chapters 60–63) may be genotoxic (see Chapters 6 and 7).
ALLERGIC REACTIONS. An allergy is an adverse reaction, mediated by the immune system, that results from previous sensitization to a particular chemical or to 1 that is structurally similar. Allergic responses have been divided into 4 general categories based on the mechanism of immunological involvement.
TYPE I: ANAPHYLACTIC REACTIONS. Anaphylaxis is mediated by IgE antibodies. The Fc portion of IgE can bind to receptors on mast cells and basophils. If the Fab portion of the antibody molecule then binds an antigen, various mediators (e.g., histamine, leukotrienes, and prostaglandins) are released and cause vasodilation, edema, and an inflammatory response. The main targets of this type of reaction are the GI tract (food allergies), the skin (urticaria and atopic dermatitis), the respiratory system (rhinitis and asthma), and the vasculature (anaphylactic shock). These responses tend to occur quickly after challenge with an antigen to which the individual has been sensitized and are termed immediate hypersensitivity reactions.
TYPE II: CYTOLYTIC REACTIONS. Type II allergies are mediated by both IgG and IgM antibodies and usually are attributed to their capacity to activate the complement system. The major target tissues for cytolytic reactions are the cells in the circulatory system. Examples of type II allergic responses include penicillin-induced hemolytic anemia, quinidine-induced thrombocytopenic purpura, and sulfonamide-induced granulocytopenia. These autoimmune reactions to drugs usually subside within several months after removal of the offending agent.
TYPE III: ARTHUS REACTIONS. Type III allergic reactions are mediated predominantly by IgG; the mechanism involves the generation of antigen-antibody complexes that subsequently fix complement. The complexes are deposited in the vascular endothelium, where a destructive inflammatory response called serum sickness occurs. The clinical symptoms of serum sickness include urticarial skin eruptions, arthralgia or arthritis, lymphadenopathy, and fever. Several drugs, including commonly used antibiotics, can induce serum sickness-like reactions. These reactions usually last 6-12 days and then subside after the offending agent is eliminated.
TYPE IV: DELAYED HYPERSENSITIVITY REACTIONS. These reactions are mediated by sensitized T-lymphocytes and macrophages. When sensitized cells come in contact with antigen, an inflammatory reaction is generated by the production of lymphokines and the subsequent influx of neutrophils and macrophages. An example of type IV or delayed hypersensitivity is the contact dermatitis caused by poison ivy.
IDIOSYNCRATIC REACTIONS; PHARMACOGENETIC CONTRIBUTIONS. Idiosyncrasy is an abnormal reactivity to a chemical that is peculiar to a given individual; the idiosyncratic response may be extreme sensitivity to low doses or extreme insensitivity to high doses of drugs.
Many interindividual differences in drug responses have a pharmacogenetic basis (see Chapter 7
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree