Chapter 4 Drug Development, Regulation, and Prescription Writing
| Abbreviations | |
|---|---|
| DEA | Drug Enforcement Administration |
| FDA | Food and Drug Administration |
| IND | Investigational new drug |
| IRB | Institutional Review Board |
| NDA | New drug application |
| NIH | National Institutes of Health |
| PMS | Postmarketing surveillance |
Therapeutic Overview
The discovery, development, and clinical introduction of new drugs is a process involving close cooperation among researchers, medical practitioners, the pharmaceutical industry, and the United States Food and Drug Administration (FDA). The drug development process begins with the synthesis or isolation of a new compound with biological activity and potential therapeutic use. This entity must then pass through preclinical, clinical, and regulatory review stages before becoming available as a therapeutically safe and effective drug. Similar governmental agencies regulate the development and distribution of drugs in other countries.
CLINICAL TESTING AND INTRODUCTION OF NEW DRUGS
These studies provide the basis for drug labeling. The completion of these clinical studies may take 3 to 10 years and typically costs more than $300 million. Only one out of every five drugs that enter clinical trials receives FDA approval. When that one drug is marketed, it often represents an average $800 million investment, because the pharmaceutical company must pay for the thousands of failed drugs that did not meet approval (Fig. 4-1). The patent protection (17 years) of new drugs may be increased on some drugs, based upon delays in FDA approval (Patent Term Restoration Act, 1984). Extensions in patent life may also occur for products that provide pediatric studies to support pediatric labeling (Best Pharmaceuticals for Children Act, 2002).
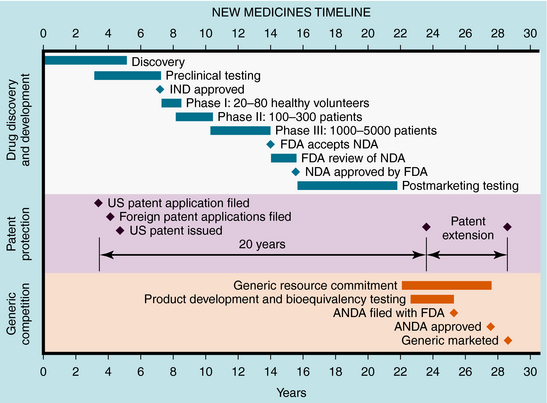
FIGURE 4–1 Stages of new drug development and the approval process, patent protection, and generic competition.
If suitable preclinical and clinical findings demonstrate efficacy with minimal toxicity, the sponsors can submit a New Drug Application (NDA) to the FDA (see Fig. 4-1). In approving an NDA, the FDA ensures the drug’s safety and effectiveness for each use. Usually, the sponsor and the FDA review the data and negotiate on the detailed information to accompany the drug for its use. This includes contraindications, precautions, side effects, dosages, routes of administration, and frequency of administration. The NDA approval process usually takes 1 to 2 years, with drugs having the greatest potential benefit given priority. Drug applications are identified and placed into specific categories under an FDA classification system (Table 4-1). Postapproval research may be requested by the FDA as a condition of new drug approval. Such research may be used to speed drug approval, uncover unexpected adverse drug reactions, and define the incidence of known drug reactions under actual clinical use.
TABLE 4–1 FDA Drug Classification System
| Designation | Meaning |
|---|---|
| AA | Drugs for AIDS or complications related to AIDS |
| P | Priority |
| S | Standard |
| O | Orphan |
AIDS, Acquired immunodeficiency syndrome.
After NDA approval, the manufacturer promotes the new drug for the approved uses described on the label. During the post-NDA approval or marketing period (Phase 4
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


