37
DNA Enveloped Viruses
CHAPTER CONTENTS
HERPESVIRUSES
The herpesvirus family contains six important human pathogens: herpes simplex virus types 1 and 2, varicella-zoster virus, cytomegalovirus, Epstein–Barr virus, and human herpesvirus 8 (the cause of Kaposi’s sarcoma).
All herpesviruses are structurally similar. Each has an icosahedral core surrounded by a lipoprotein envelope (Figure 37–1). The genome is linear double-stranded DNA. The virion does not contain a polymerase. They are large (120–200 nm in diameter), second in size only to poxviruses.
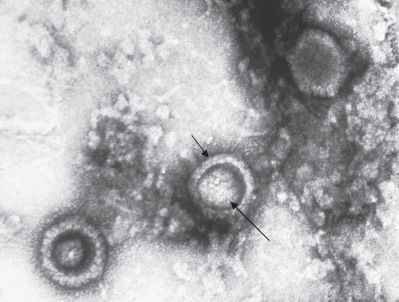
FIGURE 37–1 Herpes simplex virus (HSV)—electron micrograph. Three HSV virions are visible. Short arrow points to the envelope of an HSV virion. Long arrow points to the nucleocapsid of the virion. The dark area between the inner nucleocapsid and the outer envelope is the tegument. (Figure courtesy of Dr. John Hierholzer, Public Health Image Library, Centers for Disease Control and Prevention.)
They replicate in the nucleus, form intranuclear inclusions, and are the only viruses that obtain their envelope by budding from the nuclear membrane. The virions of herpesviruses possess a tegument located between the nucleocapsid and the envelope. This structure contains regulatory proteins, such as transcription and translation factors, which play a role in viral replication.
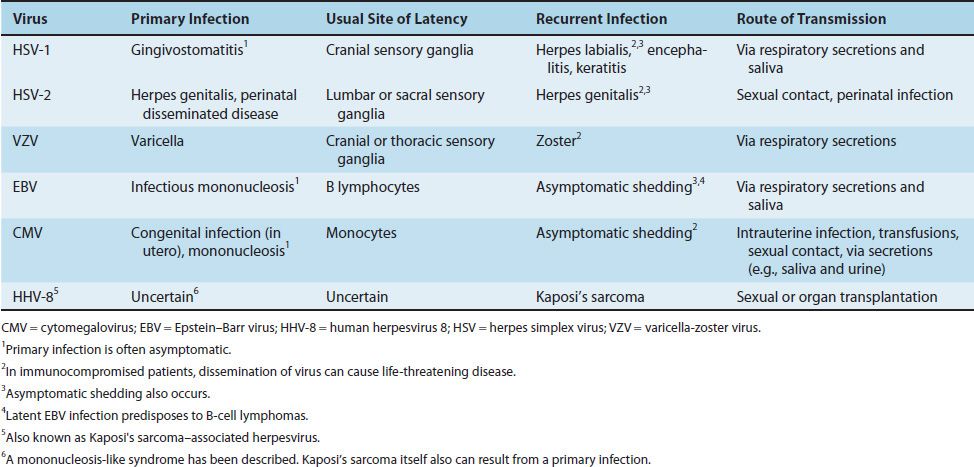
Herpesviruses are noted for their ability to cause latent infections. In these infections, the acute disease is followed by an asymptomatic period during which the virus remains in a quiescent (latent) state. When the patient is exposed to an inciting agent or immunosuppression occurs, reactivation of virus replication and disease can occur.1 With some herpesviruses (e.g., herpes simplex virus), the symptoms of the subsequent episodes are similar to those of the initial one; however, with others (e.g., varicella-zoster virus), they are different (Table 37–1).
Some information is available regarding the mechanism by which herpes simplex virus (HSV) and cytomegalovirus (CMV) initiate and maintain the latent state. Shortly after HSV infects neurons, a set of “latency-associated transcripts” (LATS) are synthesized. These noncoding, regulatory RNAs suppress viral replication. The precise mechanism by which they do so is unknown. The process by which latency is terminated and reactivation of viral replication occurs is unclear, but various triggers such as sunlight, fever, and stress are known. CMV establishes latency by producing microRNAs that inhibit the translation of mRNAs required for viral replication. Also, the CMV genome encodes a protein and an RNA that have the ability to inhibit apoptosis in infected cells. Inhibition of apoptosis allows the infected cell to survive.
Three of the herpesviruses, HSV types 1 and 2 and varicella-zoster virus (VZV), cause a vesicular rash, both in primary infections and in reactivations. Primary infections are usually more severe than reactivations. The other two herpesviruses, CMV and Epstein–Barr virus (EBV), do not cause a vesicular rash.
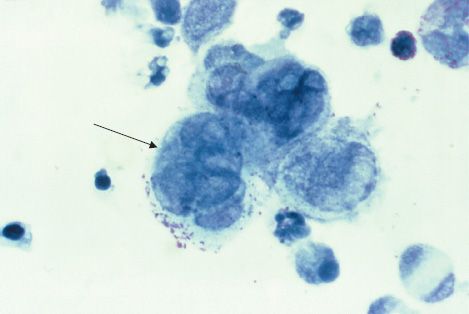
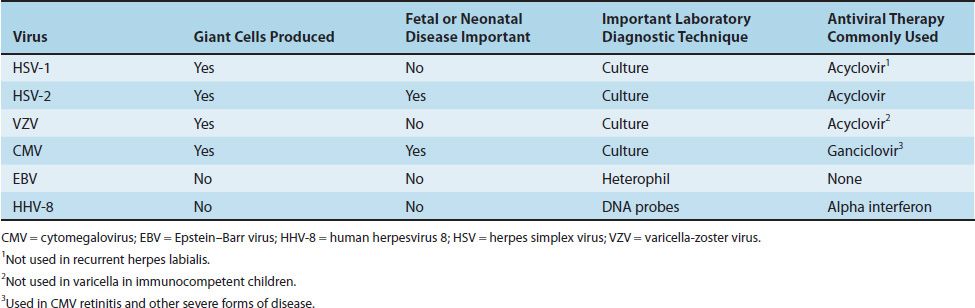
Four herpesviruses, namely HSV types 1 and 2, VZV, and CMV, induce the formation of multinucleated giant cells, which can be seen microscopically in the lesions. The importance of giant cells is best illustrated by the Tzanck smear, which reveals multinucleated giant cells in a smear taken from the painful vesicles of the genitals caused by HSV type 2 (Figure 37–2).
FIGURE 37–2 Herpes simplex virus type 2—multinucleated giant cells in Tzanck smear. Arrow points to a multinucleated giant cell with approximately eight nuclei. (Figure courtesy of Dr. Joe Miller, Public Health Image Library, Centers for Disease Control and Prevention.)
The herpesvirus family can be subdivided into three categories based on the type of cell most often infected and the site of latency. The alpha herpesviruses, consisting of HSV types 1 and 2 and VZV, infect epithelial cells primarily and cause latent infection in neurons. The beta herpesviruses, consisting of CMVs and human herpesvirus 6, infect and become latent in a variety of tissues. The gamma herpesviruses, consisting of EBV and human herpesvirus 8 (HHV-8, Kaposi’s sarcoma–associated virus), infect and become latent primarily in lymphoid cells. Table 37–2 describes some important clinical features of the common herpesviruses.
Certain herpesviruses are associated with or cause cancer in humans (e.g., Epstein–Barr virus is associated with Burkitt’s lymphoma and nasopharyngeal carcinoma, and human herpesvirus 8 causes Kaposi’s sarcoma). Several herpesviruses cause cancer in animals (e.g., leukemia in monkeys and lymphomatosis in chickens) (see Chapter 43).
HERPES SIMPLEX VIRUSES (HSV)
HSV type 1 (HSV-1) and type 2 (HSV-2) are distinguished by two main criteria: antigenicity and location of lesions. Lesions caused by HSV-1 are, in general, above the waist, whereas those caused by HSV-2 are below the waist. Table 37–3 describes some important differences between the diseases caused by HSV-1 and HSV-2.
Diseases
HSV-1 causes acute gingivostomatitis, recurrent herpes labialis (cold sores), keratoconjunctivitis (keratitis), and encephalitis, primarily in adults. HSV-2 causes herpes genitalis (genital herpes), neonatal encephalitis and other forms of neonatal herpes, and aseptic meningitis. Infection by HSV-1 or HSV-2 is a common cause of erythema multiforme.
Important Properties
HSV-1 and HSV-2 are structurally and morphologically indistinguishable. They can, however, be differentiated by the restriction endonuclease patterns of their genome DNA and by type-specific monoclonal antisera against glycoprotein G. Humans are the natural hosts of both HSV-1 and HSV-2.
Summary of Replicative Cycle
The cycle begins when HSV-1 binds first to heparan sulfate on the cell surface and then to a second receptor, nectin. Following fusion of the viral envelope with the cell membrane, the nucleocapsid and the tegument proteins are released into the cytoplasm. The viral nucleocapsid is transported to the nucleus, where it docks to a nuclear pore and the genome DNA enters the nucleus along with tegument protein VP16. The linear genome DNA now becomes circular. VP16 interacts with cellular transcription factors to activate transcription of viral immediate early (IE) genes by host cell RNA polymerase. IE mRNA is translated into IE proteins that regulate the synthesis of early proteins such as the DNA polymerase that replicates the genome and thymidine kinase. These two proteins are important because they are involved in the action of acyclovir, which is the most important drug effective against HSV.
Note that early protein synthesis by HSV can be subdivided into two categories: immediate early and early. Immediate early proteins are those whose mRNA synthesis is activated by a protein brought in by the incoming parental virion (i.e., no new viral protein synthesis is required for the production of the five immediate early proteins). The early proteins, on the other hand, do require the synthesis of new viral regulatory proteins to activate the transcription of their mRNAs.
The viral DNA polymerase replicates the genome DNA, at which time early protein synthesis is shut off and late protein synthesis begins. These late, structural proteins are transported to the nucleus, where virion assembly occurs. The virion obtains its envelope by budding through the nuclear membrane and exits the cell via tubules or vacuoles that communicate with the exterior.
In latently infected cells, such as HSV-infected neurons, circular HSV DNA resides in the nucleus and is not integrated into cellular DNA. Transcription of HSV DNA is limited to a few latency-associated transcripts (LATS). These noncoding, regulatory RNAs suppress viral replication. Reactivation of viral replication can occur at a later time when the genes encoding LATS are excised.
Transmission & Epidemiology
HSV-1 is transmitted primarily in saliva, whereas HSV-2 is transmitted by sexual contact. As a result, HSV-1 infections occur mainly on the face, whereas HSV-2 lesions occur in the genital area. However, oral–genital sexual practices can result in HSV-1 infections of the genitals and HSV-2 lesions in the oral cavity (this occurs in about 10%–20% of cases). Although transmission occurs most often when active lesions are present, asymptomatic shedding of both HSV-1 and HSV-2 does occur and plays an important role in transmission.
The number of HSV-2 infections has markedly increased in recent years, whereas that of HSV-1 infections has not. Roughly 80% of people in the United States are infected with HSV-1, and 40% have recurrent herpes labialis. Most primary infections by HSV-1 occur in childhood, as evidenced by the early appearance of antibody. In contrast, antibody to HSV-2 does not appear until the age of sexual activity.
Pathogenesis & Immunity
The virus replicates in the skin or mucous membrane at the initial site of infection, and then migrates up the neuron by retrograde axonal flow and becomes latent in the sensory ganglion cells. In general, HSV-1 becomes latent in the trigeminal ganglia, whereas HSV-2 becomes latent in the lumbar and sacral ganglia. During latency, most—if not all—viral DNA is located in the cytoplasm rather than integrated into nuclear DNA. The virus can be reactivated from the latent state by a variety of inducers (e.g., sunlight, hormonal changes, trauma, stress, and fever), at which time it migrates down the neuron and replicates in the skin, causing lesions.
The typical skin lesion is a vesicle that contains serous fluid filled with virus particles and cell debris. When the vesicle ruptures, virus is liberated and can be transmitted to other individuals. Multinucleated giant cells are typically found at the base of herpesvirus lesions.
Immunity is type-specific, but some cross-protection exists. However, immunity is incomplete, and both reinfection and reactivation occur in the presence of circulating IgG. Cell-mediated immunity is important in limiting herpesviruses, because its suppression often results in reactivation, spread, and severe disease.
Clinical Findings
HSV-1 causes several forms of primary and recurrent disease:
(1) Gingivostomatitis occurs primarily in children and is characterized by fever, irritability, and vesicular lesions in the mouth. The primary disease is more severe and lasts longer than recurrences. The lesions heal spontaneously in 2 to 3 weeks. Many children have asymptomatic primary infections.
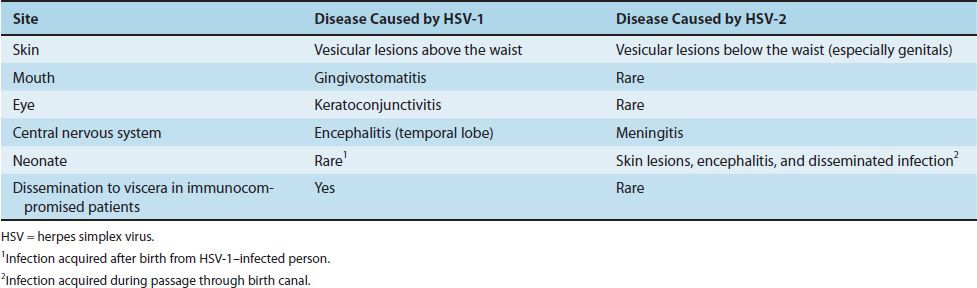
(2) Herpes labialis (fever blisters or cold sores) is the milder, recurrent form and is characterized by crops of vesicles, usually at the mucocutaneous junction of the lips or nose (Figure 37–3). Recurrences frequently reappear at the same site.
FIGURE 37–3 Herpes labialis—note vesicles on upper lip adjacent to the vermillion border of the lip caused by herpes simplex virus type 1. (Figure courtesy of Jack Resneck, Sr., MD.)
(3) Keratoconjunctivitis is characterized by corneal ulcers and lesions of the conjunctival epithelium. Recurrences can lead to scarring and blindness.
(4) Encephalitis caused by HSV-1 is characterized by a necrotic lesion in one temporal lobe. Fever, headache, vomiting, seizures, and altered mental status are typical clinical features. The onset may be acute or protracted over several days. The disease occurs as a result of either a primary infection or a recurrence. Magnetic resonance imaging often reveals the lesion. Examination of the spinal fluid typically shows a moderate increase of lymphocytes, a moderate elevation in the amount of protein, and a normal amount of glucose. HSV-1 encephalitis has a high mortality rate and causes severe neurologic sequelae in those who survive.
(5) Herpetic whitlow is a pustular lesion of the skin of the finger or hand. It can occur in medical personnel as a result of contact with patient’s lesions.
(6) Herpes gladiatorum, as the name implies, occurs in wrestlers and others who have close body contact. It is caused primarily by HSV-1 and is characterized by vesicular lesions on the head, neck, and trunk.
(7) Eczema herpeticum (Kaposi’s varicelliform eruption) is an infection of the skin of a patient with atopic dermatitis. Vesicular lesions are seen at the site of the atopic dermatitis (eczema). Most cases occur in children.
(8) Disseminated infections, such as esophagitis and pneumonia, occur in immunocompromised patients with depressed T-cell function.
HSV-2 causes several diseases, both primary and recurrent:
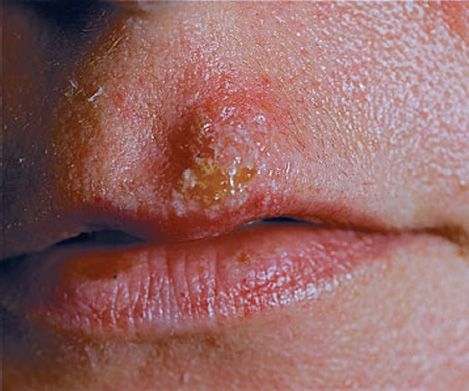
(1) Genital herpes is characterized by painful vesicular lesions of the male and female genitals and anal area (Figure 37–4). The lesions are more severe and protracted in primary disease than in recurrences. Primary infections are associated with fever and inguinal adenopathy. Asymptomatic infections occur in both men (in the prostate or urethra) and women (in the cervix) and can be a source of infection of other individuals. Many infections are asymptomatic (i.e., many people have antibody to HSV-2 but have no history of disease).
FIGURE 37–4 Herpes genitalis—note vesicles on shaft of penis caused by herpes simplex virus type 2. (Figure courtesy of Jack Resneck, Sr., MD.)
Approximately 80% to 90% of herpes genitalis cases are caused by HSV-2. The remainder are caused by HSV-1 as a result of oral–genital contact. The clinical importance of this is that suppressive chemoprophylaxis for HSV-2 lesions should be considered because lesions caused by HSV-2 are more likely to recur than lesions caused by HSV-1.
(2) Neonatal herpes originates chiefly from contact with vesicular lesions within the birth canal. In some cases, although there are no visible lesions, HSV-2 is shed into the birth canal (asymptomatic shedding) and can infect the child during birth. Neonatal herpes varies from severe disease (e.g., disseminated lesions or encephalitis) to milder local lesions (skin, eye, mouth) to asymptomatic infection. Neonatal disease may be prevented by performing cesarean section on women with either active lesions or positive viral cultures. Both HSV-1 and HSV-2 can cause severe neonatal infections that are acquired after birth from carriers handling the child. Despite their association with neonatal infections, neither HSV-1 nor HSV-2 causes congenital abnormalities to any significant degree.
Serious neonatal infection is more likely to occur when the mother is experiencing a primary herpes infection than a recurrent infection for two reasons: (1) the amount of virus produced during a primary infection is greater than during a secondary infection, and (2) mothers who have been previously infected can pass IgG across the placenta, which can protect the neonate from serious disseminated infection.
(3) Aseptic meningitis caused by HSV-2 is usually a mild, self-limited disease with few sequelae.
Both HSV-1 and HSV-2 infections are associated with erythema multiforme. The rash of erythema multiforme appears as a central red area surrounded by a ring of normal skin outside of which is a red ring (“target” or “bull’s eye” lesion). The lesions are typically macular or papular and occur symmetrically on the trunk, hands, and feet. The rash is thought to be an immune-mediated reaction to the presence of HSV antigens. Acyclovir is useful in preventing recurrent episodes of erythema multiforme, probably by reducing the amount of HSV antigens. Many drugs, especially sulfonamides among the antimicrobial drugs, commonly cause erythema multiforme. Other prominent infectious causes include Mycoplasma pneumoniae and viruses such as hepatitis B virus and hepatitis C virus.
Erythema multiforme major, also known as Stevens-Johnson syndrome, is characterized by fever, erosive oral lesions, and extensive desquamating skin lesions. M. pneumoniae infection is the most common infectious cause of Stevens-Johnson syndrome.
Laboratory Diagnosis
An important diagnostic procedure is isolation of the virus from the lesion by growth in cell culture. The typical cytopathic effect occurs in 1 to 3 days, after which the virus is identified by fluorescent antibody staining of the infected cells or by detecting virus-specific glycoproteins in enzyme-linked immunosorbent assays (ELISAs). HSV-1 can be distinguished from HSV-2 by using monoclonal antibody against glycoprotein G often in an ELISA test.
A rapid presumptive diagnosis can be made from skin lesions by using the Tzanck smear, in which cells from the base of the vesicle are stained with Giemsa stain. The presence of multinucleated giant cells suggests herpesvirus infection (Figure 37–2).
If herpes encephalitis is suspected, a rapid diagnosis can be made by detecting HSV DNA in the spinal fluid by using a polymerase chain reaction (PCR) assay. The PCR assay is more sensitive than viral culture. The diagnosis of neonatal herpes infection typically involves the use of viral cultures or PCR assay.
Serologic tests such as the neutralization test can be used in the diagnosis of primary infections because a significant rise in antibody titer is readily observed. However, they are of no use in the diagnosis of recurrent infections because many adults already have circulating antibodies, and recurrences rarely cause a rise in antibody titer.
Treatment
Acyclovir (acycloguanosine, Zovirax) is the treatment of choice for encephalitis and systemic disease caused by HSV-1. It is also useful for the treatment of primary and recurrent genital herpes; it shortens the duration of the lesions and reduces the extent of shedding of the virus but does not cure the latent state. Acyclovir is also used to treat neonatal infections caused by HSV-2. Mutants of HSV-1 resistant to acyclovir have been isolated from patients; foscarnet can be used in these cases.
For HSV-1 eye infections, other nucleoside analogues (e.g., trifluridine [Viroptic]) are used topically. Oral acyclovir is also used for HSV keratitis. Penciclovir (a derivative of acyclovir) or docosanol (a long-chain saturated alcohol) can be used to treat recurrences of orolabial HSV-1 infections in immunocompetent adults. Valacyclovir (Valtrex) and famciclovir (Famvir) are used in the treatment of genital herpes and in the suppression of recurrences.
Note that no drug treatment of the primary infection prevents recurrences; drugs have no effect on the latent state, but prophylactic, long-term administration of acyclovir, valacyclovir, or famciclovir can suppress clinical recurrences.
Prevention
Valacyclovir (Valtrex) and famciclovir (Famvir) are used in the suppression of recurrent lesions, especially in those with frequent recurrences caused by HSV-2. Suppressive chemoprophylaxis also reduces shedding of the virus and, as a result, transmission to others. Prevention also involves avoiding contact with the vesicular lesion or ulcer. Cesarean section is recommended for women who are at term and who have genital lesions or positive viral cultures. Circumcision reduces the risk of infection by HSV-2. There is no vaccine against HSV-1 or HSV-2.
VARICELLA-ZOSTER VIRUS (VZV)
Disease
Varicella (chickenpox) is the primary disease; zoster (shingles) is the recurrent form.
Important Properties
VZV is structurally and morphologically similar to other herpesviruses but is antigenically different. It has a single serotype. The same virus causes both varicella and zoster. Humans are the natural hosts.
Summary of Replicative Cycle
The cycle is similar to that of HSV (see page 284).
Transmission & Epidemiology
The virus is transmitted by respiratory droplets and by direct contact with the lesions. Varicella is a highly contagious disease of childhood; more than 90% of people in the United States have antibody by age 10 years. Varicella occurs worldwide. Prior to 2001, there were more cases of chickenpox than any other notifiable disease, but the widespread use of the vaccine has significantly reduced the number of cases.
There is infectious VZV in zoster vesicles. This virus can be transmitted, usually by direct contact, to children and can cause varicella. The appearance of either varicella or zoster in a hospital is a major infection control problem because the virus can be transmitted to immunocompromised patients and cause life-threatening disseminated infection.
Pathogenesis & Immunity
VZV infects the mucosa of the upper respiratory tract, and then spreads via the blood to the skin, where the typical vesicular rash occurs. Multinucleated giant cells with intranuclear inclusions are seen in the base of the lesions. The virus infects sensory neurons and is carried by retrograde axonal flow into the cells of the dorsal root ganglia, where the virus becomes latent.
In latently infected cells, VZV DNA is located in the nucleus and is not integrated into cellular DNA. Later in life, frequently at times of reduced cell-mediated immunity or local trauma, the virus is activated and causes the vesicular skin lesions and nerve pain of zoster.
Immunity following varicella is lifelong: A person gets varicella only once, but zoster can occur despite this immunity to varicella. Zoster usually occurs only once. The frequency of zoster increases with advancing age, perhaps as a consequence of waning immunity.
Clinical Findings
Varicella
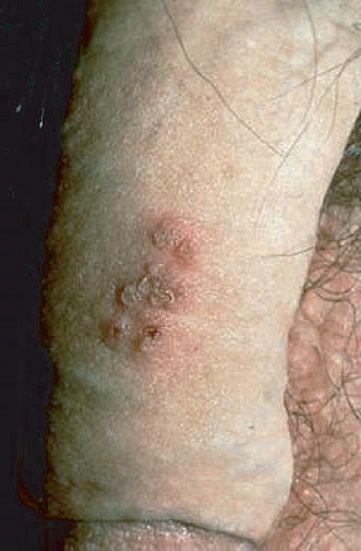
After an incubation period of 14 to 21 days, brief prodromal symptoms of fever and malaise occur. A papulovesicular rash then appears in crops on the trunk and spreads to the head and extremities (Figure 37–5). The rash evolves from papules to vesicles, pustules, and, finally, crusts. Itching (pruritus) is a prominent symptom, especially when vesicles are present. Varicella is mild in children but more severe in adults. Varicella pneumonia and encephalitis are the major rare complications, occurring more often in adults. Reye’s syndrome, characterized by encephalopathy and liver degeneration, is associated with VZV and influenza B virus infection, especially in children given aspirin. Its pathogenesis is unknown.
FIGURE 37–5 Varicella (chickenpox)—note vesicles on an erythematous base caused by varicella-zoster virus. (Figure courtesy of Richard P. Usatine, MD, and The Color Atlas of Family Medicine.)
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree