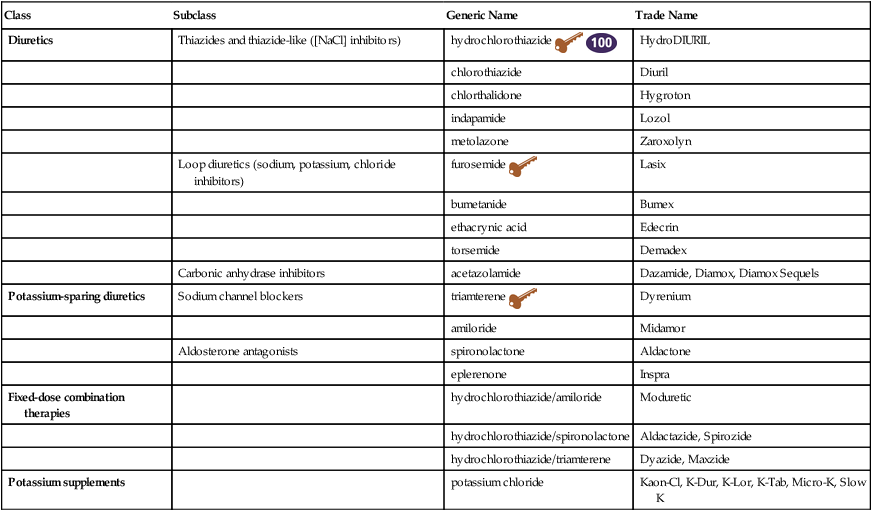
Chapter 32 The four major subclasses of diuretics—thiazides, loop, carbonic anhydrase inhibitors, and potassium-sparing diuretics (Table 32-1)—act by decreasing sodium reabsorption at different sites along the nephron. The four classes differ in terms of the specific site of action in the nephron. The ability to augment urinary losses of sodium and water is useful in the treatment of hypertension, heart failure, renal failure, and cirrhosis. A large number of thiazide diuretics are available; only the five most commonly used are discussed here. The use of potassium supplementation is discussed separately at the end of the chapter. All diuretics are now available in generic forms. TABLE 32-1 The sites of action of the different types of diuretics are outlined in Figure 32-1 and Table 32-1. The ability of each type of diuretic to increase urinary sodium excretion depends on two factors: the amount of sodium reabsorbed at its site of action and the ability of more distal sites to reclaim that sodium. Carbonic anhydrase inhibitors act on the proximal tubule, where up to 65% of sodium is reabsorbed. However, these drugs have limited clinical usefulness as diuretics because sodium lost at this site is effectively reclaimed at more distal sites along the nephron. Loop diuretics act in the ascending limb of the loop of Henle, where 25% of sodium is normally reclaimed. Thiazide diuretics act on the distal tubule, where 3% to 5% of sodium is reclaimed. The collecting duct is the site of action of the potassium-sparing diuretics. Normally, only 1% to 2% of the sodium is reabsorbed at this site. These drugs, as their group name implies, limit urinary losses of potassium. Twenty percent of filtered sodium is reabsorbed in the loop of Henle. A cotransporter in the ascending limb of the loop of Henle and in the macula densa cells of the early distal tubule moves one molecule of sodium and potassium and two molecules of chloride from the tubular lumen into the tubular epithelial cells (see Figure 32-1). Most reabsorbed potassium then moves back out of the cell into the tubular lumen via potassium channels. Loop diuretics inhibit this cotransporter and are able to excrete up to 25% of the filtered sodium. Ototoxicity, caused by intravenous loop diuretic therapy, is thought to be related to inhibition of this cotransporter in the inner ear. Approximately 50% to 75% of filtered sodium is reabsorbed in the proximal tubule. However, the diuretic response is generally weak because most filtered sodium is reclaimed by both the loop of Henle and the distal nephron. An important sodium transport pathway at this site is the sodium/hydrogen (sodium/H) exchanger that is located in the luminal membrane of the proximal tubular cells (see Figure 32-1). An important factor in maintenance of sodium/H exchange is the removal of hydrogen ions from the tubular lumen. If hydrogen ions accumulate, then the activity of the sodium/H exchanger is slowed. Carbonic anhydrase facilitates the removal of luminal hydrogen ions by promoting the dissociation of carbonic acid, H2CO3 (formed by the association of filtered bicarbonate with the hydrogen ions secreted into the tubular lumen by the sodium/H exchanger), to yield carbon dioxide and water. This process of carbonic acid formation and dissociation maintains low luminal hydrogen ion levels and thus allows the sodium/H exchanger to continue to reabsorb sodium. If carbonic anhydrase is inhibited, luminal hydrogen ion concentrations rise and the activity of the sodium/H exchanger is inhibited. The carbonic anhydrase inhibitors are relatively weak diuretics because of the ability of more distal sites to increase their reabsorption of sodium. An important consequence of carbonic anhydrase inhibition is its interference with bicarbonate reabsorption (see Figure 32-1). The distal nephron sites are not able to reclaim all of this additional bicarbonate; as a result, bicarbonate is lost into the urine. This loss of bicarbonate can be an advantage in the treatment of individuals with severe metabolic alkalosis, in whom this bicarbonate loss can limit alkalemia. However, loss of urinary bicarbonate can also lead to severe metabolic acidosis. • Chobanian AV et al: The seventh report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure: the JNC 7 report, JAMA 289:2560, 2003. • Adams KF et al: Executive summary: HFSA 2006 comprehensive heart failure practice guideline, J Cardiac Failure 12:10-38, 2006 (full 112-page guidelines available online at www.hfsa.org). For diuretic resistance, evaluate and treat these factors: patient nonadherence (either not taking drug or high NaCl intake), HF, renal failure, increased renal insufficiency, nephrotic syndrome, and cirrhosis. Drugs that may cause diuretic resistance include NSAIDs, captopril, cimetidine, and some antihypertensives. (See Boxes 32-1 and 32-2.)
Diuretics
Class
Subclass
Generic Name
Trade Name
Diuretics
Thiazides and thiazide-like ([NaCl] inhibitors)
hydrochlorothiazide ![]()
![]()
HydroDIURIL
chlorothiazide
Diuril
chlorthalidone
Hygroton
indapamide
Lozol
metolazone
Zaroxolyn
Loop diuretics (sodium, potassium, chloride inhibitors)
furosemide ![]()
Lasix
bumetanide
Bumex
ethacrynic acid
Edecrin
torsemide
Demadex
Carbonic anhydrase inhibitors
acetazolamide
Dazamide, Diamox, Diamox Sequels
Potassium-sparing diuretics
Sodium channel blockers
triamterene ![]()
Dyrenium
amiloride
Midamor
Aldosterone antagonists
spironolactone
Aldactone
eplerenone
Inspra
Fixed-dose combination therapies
hydrochlorothiazide/amiloride
Moduretic
hydrochlorothiazide/spironolactone
Aldactazide, Spirozide
hydrochlorothiazide/triamterene
Dyazide, Maxzide
Potassium supplements
potassium chloride
Kaon-Cl, K-Dur, K-Lor, K-Tab, Micro-K, Slow K
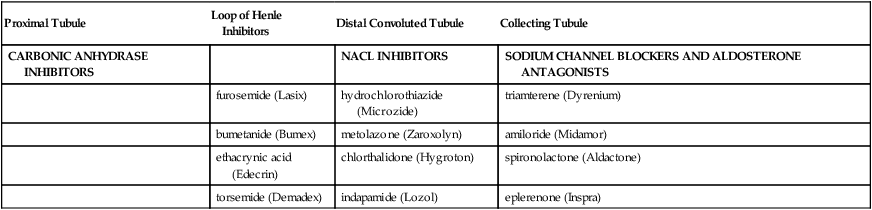
Diuretics
Proximal Tubule
Loop of Henle Inhibitors
Distal Convoluted Tubule
Collecting Tubule
CARBONIC ANHYDRASE INHIBITORS
NACL INHIBITORS
SODIUM CHANNEL BLOCKERS AND ALDOSTERONE ANTAGONISTS
furosemide (Lasix)
hydrochlorothiazide (Microzide)
triamterene (Dyrenium)
bumetanide (Bumex)
metolazone (Zaroxolyn)
amiloride (Midamor)
ethacrynic acid (Edecrin)
chlorthalidone (Hygroton)
spironolactone (Aldactone)
torsemide (Demadex)
indapamide (Lozol)
eplerenone (Inspra)
Mechanism of Action
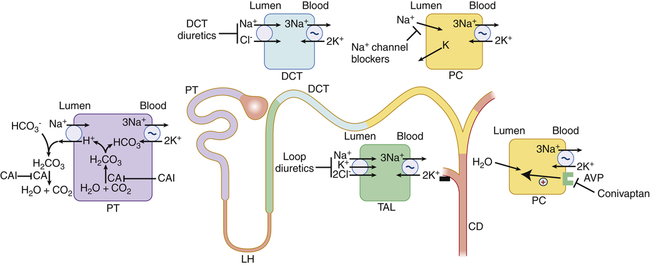
Spironolactone (not shown) is a competitive aldosterone antagonist that acts primarily in the collecting duct. As the most distal part of the nephron, it exhibits the least amount of reabsorption. CA, Carbonic anhydrase; CAI, carbonic anhydrase inhibitor; CD, collecting duct; DCT, distal convoluted tubule; DT, distal tubule; LH, loop of Henle; PC, principal cell; PT, proximal tubule; TAL, thick ascending limb; indicates primary active transport. (From Greenberg A: Primer on Kidney Diseases, ed 5, Philadelphia, 2010, Saunders.)
Loop Diuretics
Carbonic Anhydrase Inhibitors
Treatment Principles
Standardized Guidelines
Pharmacologic Treatment
< div class='tao-gold-member'>
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


Diuretics
Only gold members can continue reading. Log In or Register to continue
