Introduction
Disorders of the female reproductive system can occur as a result of disease in one of the many varied reproductive organs: the ovaries, the fallopian tubes, the uterus, the cervix, the vagina, or the breast. During the reproductive years, these disorders often present as altered menstruation, pelvic pain, or infertility. Cancers arising in these tissues occur more often in the late reproductive or menopausal years. Unfortunately, for several reasons, they often have high mortality rates and a high incidence of metastases when they are diagnosed. Some organs are located deep and are relatively inaccessible to palpation (ovaries). Others have few sensory nerves (ovary, fallopian tubes) and hence remain asymptomatic. Additionally, the breasts have large amounts of adipose tissue, which can make early detection of breast cancer difficult. The one exception is the uterine cervix. It has easy access to surveillance with use of the Papanicolaou smear and human papillomavirus (HPV) screening, which have led to a dramatically reduced mortality rate of cervical cancer.
Disorders of the female reproductive system can also occur as a result of disease in other organs whose function affects reproductive organs (eg, the brain, hypothalamus, pituitary, thyroid, adrenals, kidney, and liver). Presentation of these disorders is typically painless.
Conversely, disorders of the reproductive system can cause disorders in other tissues. Ovarian hormones are necessary for the maintenance and health of most tissues in women. Alterations in these hormones can lead to osteoporosis (loss of bone mass), atrophy and inflammation of estrogen-deprived tissues (eg, atrophic vaginitis), atherogenesis and alterations in cardiovascular compliance, and an increased risk of some forms of cancer (eg, endometrial carcinoma as a consequence of estrogen excess and progesterone deficiency). Dysfunction of the reproductive system also can contribute to unique variants of systemic disorders, such as gestational diabetes and the hypertensive syndrome of preeclampsia-eclampsia.
Checkpoint
1. How do female reproductive system disorders present during the reproductive years?
2. To what might you ascribe the lack of reduction in mortality rate from ovarian cancer in contrast to cervical cancer?
3. What are some consequences of reproductive system dysfunction?
Normal Structure & Function of the Female Reproductive Tract
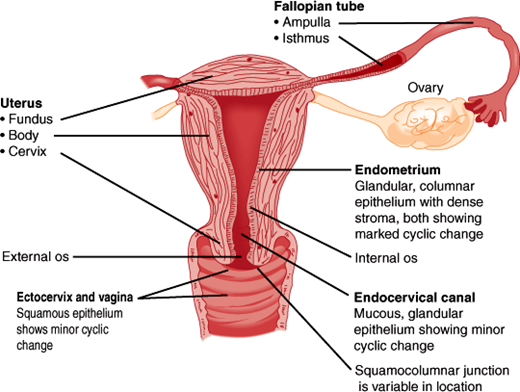
The reproductive pelvic organs include the vagina, cervix, uterus, fallopian tubes, and ovaries (Figure 22–1). The two ovaries contain thousands of follicles, each with an oocyte surrounded by a layer of granulosa cells and thecal cells. These supporting cells produce steroids and paracrine products important in follicular maturation and coordination of events in reproduction. The fallopian tubes, which are open to the peritoneal space, connect the ovaries to the uterus. The uterus contains an internal hormone-sensitive mucosal lining, the endometrium. During nonpregnant cycles, menstrual bleeding occurs as the monthly culmination of endometrial growth, differentiation, and sloughing in response to changes in blood levels of estrogen and progesterone (Figure 22–2). During pregnancy, the endometrium produces a wide variety of endocrine and paracrine products, which promote embryonic implantation (Table 22–1). Surrounding the endometrium is the smooth muscle layer of the uterus, the myometrium. Contractions of the myometrium lead to menstrual cramps or expel the fetus at parturition. The cervix is contiguous with the uterus and is the conduit for passage of menses or the fetus into the vagina, the muscular tube opening into the vulva.
Figure 22–2
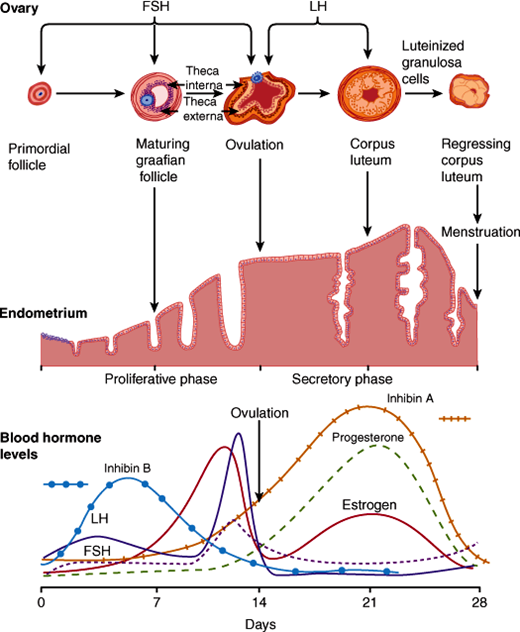
Changes in the ovary, endometrium, and blood hormone levels during the menstrual cycle. Anti-müllerian hormone levels stay constant during the cycle. FSH, follicle-stimulating hormone; LH, luteinizing hormone. (Redrawn, with permission, from Chandrasoma P et al. Concise Pathology, 3rd ed. Originally published by Appleton & Lange. Copyright © 1998 by The McGraw-Hill Companies, Inc.)
| Lipids | Cytokines | Peptides and others |
|---|---|---|
| Prostaglandins | Interleukin-1α | Prolactin |
| Thromboxanes | Interleukin-1β | Relaxin |
| Leukotrienes | Interleukin-6 | Renin |
| Interleukin-8 | Endorphin | |
| Interferon-γ | Epidermal growth factor | |
| Colony-stimulating factor-1 | IGFs | |
| VEGF | Fibroblast growth factor | |
| Platelet-derived growth factor | ||
| Transforming growth factor β | ||
| IGF-binding proteins | ||
| Glycodelin | ||
| Tumor necrosis factor | ||
| PTHrP |
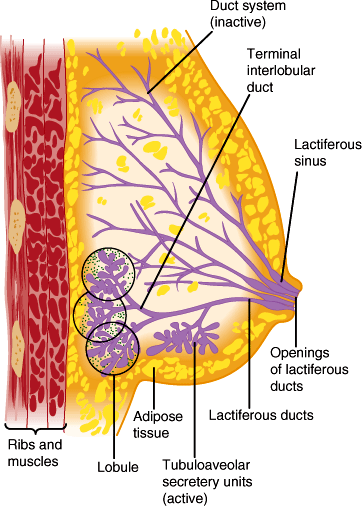
The breasts (Figure 22–3) produce, store, and eject milk upon appropriate hormonal and physical stimulation.
Figure 22–3
Schematic drawing of female breast showing the mammary glands with ducts that open in the nipple. The outlines of the lobules do not exist in vivo but are shown for instructional purposes. The stippling indicates loose intralobular connective tissue. (Redrawn, with permission, from Junqueira LC et al. Basic Histology, 10th ed. McGraw-Hill, 2003.)
During embryonic development, the primordial gametes originate in the endoderm of the yolk sac, allantois, and hindgut and migrate to the genital ridge by week 5 or 6 of gestation. Once at the genital ridge, they multiply and induce male or female gonads depending on the identity of the sex chromosomes.
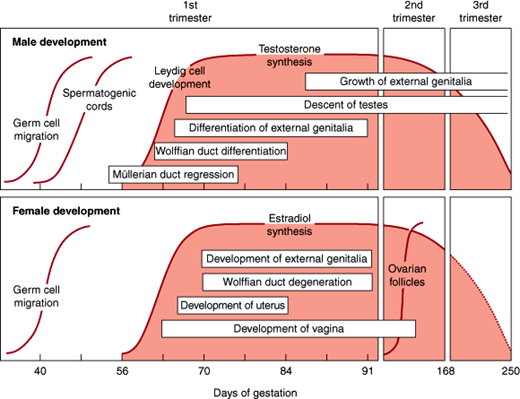
Until week 8 of gestation, the sex of the embryo cannot be determined morphologically; therefore, this period is termed the indifferent phase of sexual development. After this time, differentiation of the internal and external genitalia occurs, determining the phenotypic sex of the individual, which becomes fully developed after puberty. During embryogenesis, the internal genitalia are formed from a dual genital duct system within the urogenital ridge. The first to form is the wolffian duct, followed by the müllerian duct, which is dependent on prior wolffian duct development. After 8 weeks of gestation, production of anti-müllerian hormone by Sertoli cells in the fetal testes leads to regression of the müllerian ducts, whereas production of testosterone by the Leydig cells leads to persistence of the wolffian duct and subsequent development of the prostate, epididymis, and seminal vesicles. In the absence of these secretions, female internal reproductive organs are formed from the müllerian ducts, and the wolffian structures degenerate. Similarly, the external genitalia of males develop in the presence of dihydrotestosterone; in the absence of this hormone, the common embryologic structures give rise to female external genitalia. Exposure to androgens can result in virilization of the external genitalia of female embryos, whereas androgen deficiency results in defective male development (Figure 22–4). Therefore, the male phenotype is induced while no ovarian secretions are necessary for expression of the female phenotype.
During female development, the female ovaries contain about 7 million oogonia by 24 weeks of gestation. The majority of these cells die during intrauterine life, leaving only about 1 million primary oocytes at birth. This decreases to about 400,000 by puberty. The surviving oogonia are arrested at the prophase of meiosis I. Completion of the first meiotic division does not occur until the time of ovulation, and the second meiosis is completed with fertilization. Only about 400 of these oocytes mature and are released by ovulation during a woman’s lifetime; the others undergo atresia at various stages of development.
Secondary sexual characteristics develop at puberty, when maturation of the capacity for adult reproductive function occurs. The changes that occur in the brain and hypothalamus that initiate the onset of puberty involve the establishment of sleep-dependent (first) and the later truly pulsatile release of gonadotropin-releasing hormone (GnRH) from the hypothalamus. The hypothalamic kisspeptin/GPR54 ligand/receptor pair appears to be the key mediator of the onset of puberty.
The increase in GnRH leads to an increase in and a pulsatile pattern of luteinizing hormone (LH) and then follicle-stimulating hormone (FSH) secretion, hormones collectively termed gonadotropins. Before about age 10 years in girls, gonadotropin secretion is at low levels and does not display a pulsatile character. After this age, pulsatile release of GnRH begins and initiates folliculogenesis, leading to cyclic changes in estrogen and progesterone production. These changes allow estrogen-dependent tissues, such as the breasts and the endometrium, to begin their maturation. The appearance of breast development is referred to as thelarche, and the first menstrual period is termed menarche.
Checkpoint
4. What is the difference between the chromosomal, gonadal, and phenotypic sex of an individual?
5. Approximately what percentage of the total number of oocytes present in the ovaries of a female at birth completes their maturation and is released upon ovulation over the course of her reproductive life?
6. Describe some changes that occur in the female with the onset of puberty.
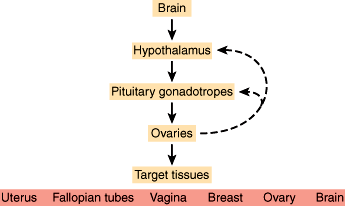
Normal female reproductive function involves coordinated interaction between the brain and ovaries under the influence of other organs such as the liver (which metabolizes hormones and makes steroid-binding globulins), adrenals, and thyroid. With this coordination, cyclic changes during the course of the menstrual cycle allow the reproductive organs to perform specific functions at different points in time to optimize the chances for successful reproduction. When these mechanisms malfunction, the result may be infertility, altered menstrual bleeding, amenorrhea, or even cancer.
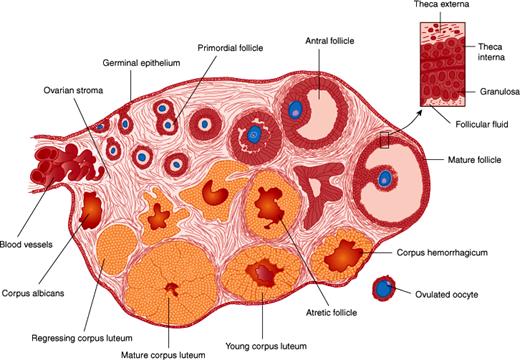
The menstrual cycle has three phases. The follicular phase typically lasts 12-14 days and culminates in the production of a mature oocyte. Initially, a cohort of follicles begins to grow, but ultimately a single dominant follicle is selected and the rest undergo a process of degeneration and apoptotic death, termed atresia (Figure 22–5). The follicular phase is followed by ovulation, in which the dominant follicle releases its mature oocyte to be transported through the uterine tubes for fertilization and subsequent implantation in a receptive uterus. The third, luteal, phase also averages 14 days and is characterized by luteinization of the ruptured follicle to produce the corpus luteum. The physiology of each of these phases in the menstrual cycle is best understood by considering different compartments: neuroendocrine, ovarian, and target tissues, most notably the uterus (Figure 22–6).
Figure 22–5
Diagram of the ovary, showing the sequential development of a follicle and the formation of a corpus luteum. An atretic follicle is shown in the center, and the structure of the wall of the mature follicle is detailed at the upper right. (Redrawn, with permission, from Gorbman A et al. Textbook of Comparative Endocrinology. Wiley, 1962.)
The neuroendocrine axis involves the brain, hypothalamus, pituitary, and the ovary. Neurons within the hypothalamus synthesize the peptide GnRH, and its secretion is modulated by endogenous opioids and corticotropin-releasing hormone (CRH). GnRH is secreted directly into the portal circulation of the pituitary in a pulsatile fashion. This pulsatility is required for proper activation of its receptor located on the gonadotropes, which are cells located in the anterior pituitary. In response, the gonadotropes secrete the polypeptides FSH and LH, collectively called gonadotropins, which stimulate the ovary to produce estrogen and inhibin. Inhibin feeds back to suppress FSH secretion but has no effect on LH. Estrogen also affects the pituitary by increasing the number of GnRH receptors and its sensitivity to GnRH stimulation. With estradiol production by the ovaries, a critical concentration is reached for a sufficient time to induce a midcycle LH surge and subsequent ovulation. After this surge, high levels of progesterone produced by the corpus luteum suppress gonadotropin release for the duration of the luteal phase.
Within the ovary, LH and FSH lead to the synthesis and secretion of steroid hormones and other paracrine/autocrine proteins, directing the maturation of a single oocyte for ovulation. During the early follicular phase, FSH stimulates growth of a cohort of follicles and increases the production of inhibin and activin in granulosa cells. Activin acts in the ovary to augment the effect of FSH, increasing aromatase activity and increasing production of FSH and LH receptors. LH stimulates the production of androgens in the thecal cells, which is augmented by inhibin. Androgens diffuse into the granulosa cells to be converted to estrogens through the enzymatic reaction of aromatization. As the follicular phase progresses, inhibin production comes under the control of LH, and the increasing amounts of inhibin lead to further conversion of androgens to produce the high levels of estrogen needed for the LH surge.
The midcycle LH surge triggers the final steps of oocyte maturation and resumption of meiosis within the dominant oocyte. Changes in prostaglandins and proteases allow digestion of the follicular wall leading to oocyte extrusion and ovulation. The follicular cells remaining after ovulation develop into a structure called the corpus luteum, which synthesizes and releases large amounts of both estradiol and progesterone. Continued secretion from the corpus luteum requires LH (or human chorionic gonadotropin [hCG], as discussed below) stimulation; in its absence, degeneration occurs.
The uterine compartment reacts to the steroids produced from the ovaries throughout the menstrual cycle. During the follicular phase, the endometrium proliferates under the influence of estrogen, creating straight glands with thin secretions and microvascular proliferation. During the luteal phase, the high levels of estradiol and progesterone promote maturation of the endometrium, which develops tortuous glands engorged with thick secretions and proteins (Figure 22–2). Additionally, the endometrium secretes a number of endocrine and paracrine factors (Table 22–1). These changes optimize the environment for implantation. In the absence of pregnancy, the corpus luteum cannot sustain the high levels of progesterone production and the endometrial vasculature cannot be maintained. This leads to sloughing of the endometrium and the onset of menstruation, which is marked by the nadir of estradiol and progesterone levels, ending the cycle (Figure 22–2).
Birth control pills are a pharmacologic means of preventing pregnancy by disrupting the precise timing of hormone-directed events necessary for reproduction. Current formulations include progestins alone as well as combinations of estrogens and progestins. Most preparations of estrogen and progestin block the LH surge at midcycle, thereby preventing ovulation. However, other contraceptive actions include effects on estrogen- and progesterone-sensitive tissues, such as inducing antifertility changes in cervical mucus and the endometrial lining that are unfavorable to sperm transport and embryonic implantation, respectively.
In order to mitigate the unpleasant side effects of nausea and bloating, as well as the dangerous side effect of thrombosis, the doses of estrogen and progestin have been decreased over the years. Non-oral formulations also have been developed, including long-term intrauterine and subdermal systems that deliver progestins. A transdermal patch allows absorption of estrogen and progestin without “first-pass” metabolism in the liver. Transvaginal absorption is also available with a soft ring placed monthly in the vagina. Each of these formulations provides contraceptive efficacy equal to or better than oral contraceptive pills.
Like the adrenal gland, the ovary is a steroid factory. The ovary secretes three types of steroids: progesterone, containing 21 carbons; androgens, containing 19 carbons; and estrogens, containing 18 carbons. Steroid synthesis occurs by conversion from cholesterol in a series of oxidative biochemical reactions catalyzed by enzymes in the mitochondria and the endoplasmic reticulum (see Chapter 21). The rate-limiting steps in steroid production involve the transport (StAR) and side chain cleavage of cholesterol within the mitochondrion by the enzyme cytochrome P450, family 11, subfamily A, polypeptide 1 (CYP11A1) to generate the basic steroid backbone, pregnenolone. This steroid is further modified in the endoplasmic reticulum to generate the various steroid hormones. Because steroids are synthesized by a cascade of enzyme reactions in various pathways, a block in one step (eg, resulting from a congenital enzyme defect or inhibition by certain drugs) can result in lack of synthesis of downstream products and “spillover” of precursors. Such defects are the hallmark of congenital adrenal hyperplasia (discussed in Chapter 21).
The classical mechanism of steroid hormone action involves diffusion across the plasma membrane, binding of the steroid to receptor proteins in the cytoplasm or nucleus, and, after association with chromatin, activation of transcription of selected genes by binding of the steroid-receptor complex to specific regions of DNA. In this way, the pattern of gene expression is changed in the various steroid-responsive tissues (ie, those that contain steroid receptors). Membrane-bound steroid receptors also have been shown to activate phosphorylation cascades typically regulated by growth factors.
Checkpoint
7. What are the primary target tissues for GnRH? For gonadotropins? For ovarian steroids?
8. Why is pulsatile secretion of GnRH important?
9. What are some specialized features of GnRH action?
10. What are the specific effects of gonadotropins on the ovary?
11. How does the structure of the uterine lining differ in the midfollicular versus the midluteal stages, and for what reproduction-related events is each stage optimized?
12. What products are made by a granulosa cell in the dominant follicle over the course of its lifetime?
A number of changes must occur in reproductive and other organs for establishment and successful completion of a pregnancy. Fertilization requires successful ovulation, capture of the mature oocyte by the fimbria of the fallopian tubes, and transport of the zygote to the uterus. Because fertilization usually occurs in the ampulla, it also requires effective transport of viable sperm into the distal tube.
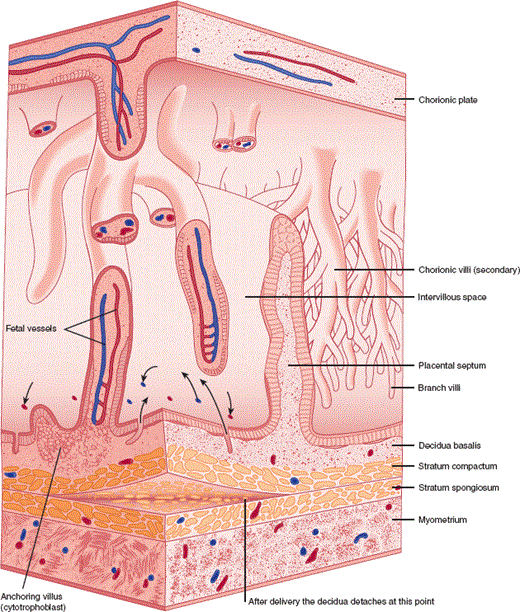
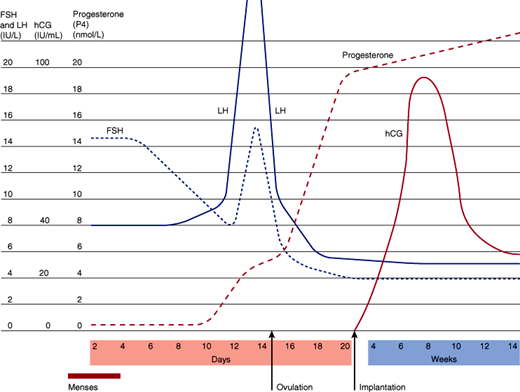
After implantation, a placenta forms consisting of two functional epithelial layers, the cytotrophoblast and the syncytiotrophoblast, as well as an adjacent maternal layer, the endometrial decidua, with its underlying mesenchymal core (Figure 22–7). The placenta allows intimate apposition of maternal and fetal circulations for exchange of nutrients, oxygen, and waste products. In addition, the placenta secretes a variety of important hormones, including an LH-like hormone termed human chorionic gonadotropin (hCG). Unlike LH secretion by the gonadotrophs of the anterior pituitary, placental secretion of hCG is neither pulsatile nor inhibited by the high levels of estrogen and progesterone. hCG maintains the corpus luteum for a period of 8–10 weeks until the full progesterone-producing capacity of the placenta has developed. At that point, hCG levels fall and the mature placenta produces progesterone from maternal cholesterol (Figure 22–8). Other factors produced by the placenta include a growth hormone–like protein human chorionic somatomammotropin (hCS), also known as placental lactogen (hPL) (Table 22–2).
Figure 22–8
Hormone production during pregnancy. (FSH, follicle-stimulating hormone; LH, luteinizing hormone; hCG, human chorionic gonadotropin.) (Redrawn and modified, with permission, from Fritz M et al. Regulation of the menstrual cycle. In: Clinical Gynecologic Endocrinology and Infertility, 8th ed. Lippincott Williams & Wilkins, 2011.)
| Fetal Compartment | Placental Compartment | Maternal Compartment |
|---|---|---|
| Alpha-fetoprotein |
|
|
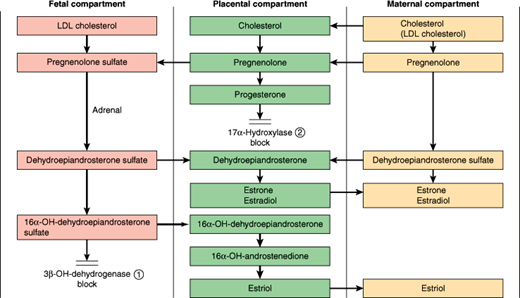
During most of pregnancy, the fetus provides the placenta with androgens, which are aromatized to make estrogens secreted into the maternal circulation (Figure 22–9). This reflects the action of a special zone in the fetal adrenal cortex engaged in androgen production. Toward the end of pregnancy, increasing ACTH secretion by the fetal pituitary triggers the fetal adrenal to produce cortisol in addition to androgen. This switch may play a role in triggering the onset of labor by modulating the expression of progesterone receptors in the myometrium.
Figure 22–9
Fetal-placental-maternal cooperation in steroidogenesis. LDL, low-density lipoprotein; 3β-Hydroxysteroid dehydrogenase, hydroxy-Δ-5-steroid dehydrogenase, 3 β- and steroid Δ-isomerase (HSD3β); 17α-Hydroxylase, 17α-hydroxylase activity of cytochrome P450, family 17, subfamily A, polypeptide 1 (CYP17A1). (Reproduced from Fritz M et al. The endocrinology of pregnancy. In: Clinical Gynecologic Endocrinology and Infertility, 8th ed. Lippincott Williams & Wilkins, 2011.)
In addition to the changes in organs with pregnancy-specific functions, physiologic changes occur in essentially every maternal organ system. These include increased blood volume (increased by more than 40% by the middle of the third trimester), increased total body water (increased by 6–8 L), and increased cardiac output because of increased stroke volume (increased by 30%) and heart rate (increased by 15%). A striking increase in minute ventilation (increased by 50% compared with nonpregnant state) without any change in respiratory rate is observed as a result of increased tidal volume (Chapter 9). Dramatic increases in renal blood flow and glomerular filtration rate (increased by 40%) are also seen. Most of these alterations are related in complex ways to the effects of hormones produced in pregnancy.
The physiological effects of various sex steroids in pregnancy are incompletely understood. The demonstrated and proposed roles of progesterone in pregnancy include (1) promotion of implantation; (2) suppression of the maternal immune response to fetal antigens, thus preventing rejection of the allogeneic fetus; (3) increased cardiovascular compliance; (4) provision of substrate for manufacture by the fetal adrenal of glucocorticoids and mineralocorticoids; (5) maintenance of myometrial quiescence through gestation; and (6) a role in parturition. Estrogens contribute to (1) volume expansion; (2) cardiac remodeling; and (3) preparative production of clotting factors, anticipating blood loss that commonly follows delivery.
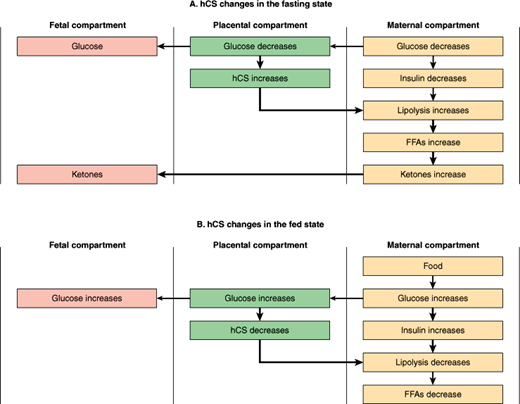
Another example of fetal-placental-maternal interactions is seen in the actions of hCS (Figure 22–10). This “counterregulatory” hormone (ie, a hormone whose actions oppose those of insulin) appears to serve as a defense against fetal hypoglycemia. From a metabolic standpoint, pregnancy is a form of “accelerated starvation” characterized by fasting hypoglycemia, as fuel substrates produced by the mother are consumed by the growing fetus. The hCS produced by the placenta in response to hypoglycemia serves to increase lipolysis, thereby raising maternal free fatty acid levels and ultimately blood glucose and ketone levels. This “diabetogenic” role of hCS is a major burden on the maternal compartment and contributes to the tendency for diabetes mellitus to emerge in susceptible individuals during pregnancy. Normally, glucose is the major fuel source for the fetus. However, in the event of glucose deprivation, ketones provide a ready emergency fuel supply (as they do in starvation) for both the mother and, via the placenta, for the fetus.
Checkpoint
13. How is the corpus luteum maintained until the placenta has developed adequately?
14. What are some possible roles of steroids during pregnancy?
15. Why is new-onset diabetes mellitus a common complication of pregnancy?
The rudiments for breast development are established during embryonic development. During puberty, rising estrogen levels stimulate breast growth as one of a number of female secondary sexual characteristics. Breast growth involves both proliferation and branching of lactiferous ducts as well as accumulation of adipose and connective tissue. In the mature breast, each terminal lactiferous duct drains clusters of tubuloalveolar secretory units lined by milk-secreting epithelial cells and is suspended in connective and adipose tissue well populated with lymphocytes. The mature female breast consists of a cluster of 15–25 lactiferous ducts, each emerging independently at the nipple (Figure 22–3). Both the pubertal and pregnant phases of breast growth require the permissive influence of glucocorticoids, thyroxine, and insulin for full development, and their actions are potentiated by estrogen and progesterone.
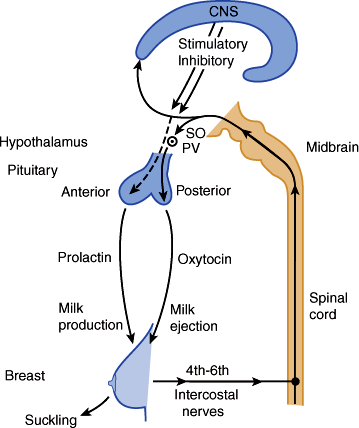
During pregnancy, prolactin, progesterone, and hCS play a dominant role in stimulating breast growth and the capacity for milk production. Actual lactation, or milk release, however, is inhibited by the high levels of placental steroids present before birth. After delivery of the placenta, estrogen and progesterone levels fall dramatically, removing this block. Maintenance of milk secretion requires the integrated action of both anterior and posterior pituitary factors (Figure 22–11) as well as interaction between the mother and infant. Suckling stimulates afferent neural pathways that suppress dopamine levels in the hypothalamus, thereby maintaining high levels of prolactin necessary for milk synthesis. At the same time, afferent sensory nerve fibers in the breast (as well as other stimuli such as the infant’s cry) stimulate synthesis, transport, and secretion of oxytocin from the posterior pituitary. Oxytocin promotes contraction of mammary myoepithelial cells, thereby triggering ejection of milk from the mammary epithelial alveoli and out the nipple.
Figure 22–11
The role of anterior and posterior pituitary factors in milk synthesis and secretion. SO, supraoptic nucleus; PV, paraventricular nucleus. (Redrawn, with permission, from Rebar RW. The breast and physiology of lactation. In: Creasy RK et al, eds. Maternal-Fetal Medicine: Principles and Practice, 4th ed. Saunders, 1999.)
Toward the end of pregnancy, there is an increase in the lymphocyte population in the vasculature and connective tissue of the breast. These lymphocytes secrete immunoglobulin A (IgA) into the local bloodstream, from which it is taken up by the mammary epithelial cells. By the process of transcytosis, IgA crosses the mammary epithelial cells into the luminal secretion (milk). This mechanism, coupled with the transplacental transport of maternal IgG, is responsible for conferring passive immunity on the newborn. The earliest mammary gland secretion after birth, termed colostrum, has particularly high immunoglobulin content.
The high level of prolactin maintained during lactation also has a contraceptive effect, primarily by inhibition of pulsatile secretion of GnRH. The precise mechanism is not known but may involve a short feedback loop by which prolactin stimulates dopamine release, which, in turn, elevates endogenous opioid release and inhibits GnRH secretion. There may also be effects of prolactin directly on the ovary that contribute to lactational anovulation and amenorrhea. However, it should be noted that the contraceptive effect of prolactin is only moderate and, therefore, of low reliability.
Checkpoint
16. Which hormones are involved in breast development?
17. Why is milk rarely secreted before parturition?
18. What is the probable mechanism of lactational amenorrhea?
Menopause is the point in a woman’s life when, as a result of exhaustion of the supply of functioning ovarian follicles, menstrual cycles cease. Ten years before menopause, at approximately age 40 years, reproductive function starts to diminish. This is manifested as a decreased frequency of ovulation and alterations in menstrual patterns. During this time, in the setting of few remaining follicles, increased GnRH-stimulated LH and FSH secretion is observed. Higher levels of estradiol circulate, particularly in the follicular phase from ages 35–48 years, and then estradiol levels sharply decline just prior to menopause. This transitory period of diminishing reproductive function approaching menopause is termed the climacteric.
During the climacteric transition, the hormonal status of women changes from a cyclic high-estrogen state to a steady-state low-estrogen postmenopausal state. This leads to vasomotor symptoms such as hot flushes (“hot flashes”), sweating, and chills. Psychologic symptoms such as irritability, tension, anxiety, and depression may also be observed. After menopause, other more gradual changes can appear. In addition to atrophy of estrogen-dependent tissues such as the vaginal epithelium, a gradual loss in bone density leading to osteoporosis can occur.
A modest degree of androgen production from thecal cells of the residual ovarian stroma continues even in the absence of follicular growth. In postmenopausal women, ovarian and adrenal androgens continue to be aromatized into estrogens by the enzyme aromatase (cytochrome P450, CYP19A1) in adipose tissue and hair follicles. The significance of peripheral aromatization in relation to severity of symptoms of menopause varies in different individuals.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree