Chapter 22 Diseases of the hair
Introduction
A distinctive phenomenon of the human evolutionary process is the loss of a great proportion of the body hair. Vestiges of hair barely remain in areas such as the scalp, axillae, genital areas, and face. Among the many species of primates, only humans are almost hairless. The time and cause of this remarkable transformation remains an enigma. It has been speculated that in the development of the human lineage there was an aquatic phase (the aquatic ape hypothesis) when the amount of body hair diminished drastically with prominent alteration in its distribution. In addition, the skin developed a thick layer of subcutaneous cell tissue similar to that present in aquatic mammals such as dolphins and whales.1 A further hypothesis has postulated the idea that the most advantageous result of this drastic evolutionary change seems to have been the acquisition of a more efficient control of body temperature through the development of the sweat apparatus; a further bonus is an improvement in interpersonal communication facilitated by the increased visibility of the facial characteristics.2 Whatever the explanation for the loss of hair in the evolutionary process may be, the truth is that the protective function of the hair layer has been lost in great part.
Paradoxically the little hair that we still have has acquired an unmeasurable importance in the realm of social interaction. Alterations in the quantity or the quality of hair often have great impact on the behavior and individual’s well-being.3 Human being’s growing preoccupation with their hair has generated a huge demand for products for the care and maintenance of the hair, significantly promoting the development of the cosmetic industry.
Diseases of the hair are usually associated with morbidity, particularly in relation to the body image and self-esteem, and the importance of this cannot be underestimated, particularly by the health professional that deals with these patients.4
The study of hair pathology has always been challenging due to the anatomical and functional complexity of the hair follicle. It consists of two major compartments: epithelial and dermal, each of which interacts both jointly and independently in a cyclical manner, under the control of a complex network of growth factors, cytokines, and hormones.5 Analysis and study of the hair follicle growth cycle is extremely informative since it represents a miniature model for studying the complex physiological mechanisms controlling cell proliferation and growth. The hair follicle is capable of sustaining a permanent cycle of growth and degeneration due to the presence of the adult stem cells that are maintained throughout our life span.6,7
Hair analysis can provide important information in forensic medicine, through the study of nuclear and mitochondrial DNA.8 The analysis of the hair is also useful in the demonstration of various poisons such as arsenic, mercury, and poisoning by trace elements.9,10 In the same way, analysis of hair follicles is a valuable tool in the diagnosis of many congenital diseases, metabolic disorders, and malformations.11
Diagnosis, history, and laboratory tests
History
Physical examination provides important information about the pattern of hair loss and whether it is nonscarring and potentially reversible (visible follicular openings) or scarring and irreversible (no visible follicular openings). Whether the hair loss is due to increased shedding or increased thinning this must be adequately investigated. Hair loss can be patterned as in androgenetic alopecia, diffuse and uniform as in telogen effluvium or patchy with surrounding normal scalp as is typical of alopecia areata. The presence or absence of active inflammation, erythema, drainage, or scaling provides important clues about the nature of the process and extent of the damage.1
Laboratory tests
Metabolic studies are sometimes essential to the investigation of hair loss. Thyroid function tests, for example, are often valuable in patients with telogen effluvium. Decreased ferritin and serum iron levels may be found in females with telogen effluvium and other types of alopecia. The significance of this finding both from the clinical and therapeutic point of view is not entirely clear.2,3 Hyperandrogenism is also sometimes responsible for hair loss in women.4 In alopecia related to autoimmune disease, particularly lupus erythematosus, investigations should include screening for autoantibodies. Direct immunofluorescence may also be rewarding in patients with scarring alopecia, particularly lupus erythematosus and lichen planus.
Hair-pluck test (trichogram)
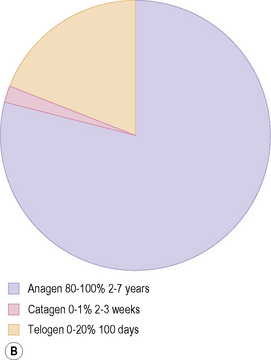
The hair-pluck test is useful to evaluate the ratio of telogen to anagen hairs. With an adequate technique, approximately 50 hairs are forcibly plucked from the scalp with a clamp or needle holder. The hair bulbs are then examined to determine the ratio of anagen hairs to telogen hairs. Normally, at least 80% of the follicles should be in the anagen stage and 20% in telogen. An increment of the hairs in telogen suggests telogen effluvium.5 Due to the pain and trauma involved in this procedure it is rarely practiced nowadays.
Hair-pull test
The hair-pull test is an easy and rapid technique which allows an accurate estimate of the amount of hair loss that the patient is experiencing. The patient must not wash his/her hair in the preceding 24 hours before the pull test. The physician grabs 20–30 hairs between the fingers and gently pulls them. Normally, with gentle traction, no more than 10%, or two to three hairs shafts, are obtained, and these by definition are in telogen. When more than three hair shafts are obtained from each pull, this is usually indicative of hair disease (telogen effluvium).6 Normally follicles in anagen are not obtained, except in children, because the attachment to the scalp is more loose.7
Daily hair shedding count
Patients are asked to collect, if possible, all the hair they shed in the shower or sink, and to brush their hair and collect the hair daily for 7 days. Commonly, between 50 and 100 telogen hairs per day are lost in a normal individual.8
Microscopic examination
Hair shaft examination
The hair samples obtained should be placed on a glass slide and fixed to the surface with transparent adhesive tape.9,10 As an alternative, the hairs can also be mounted as for routine microscopic sections with a cover-slip. This method is excellent for obtaining photomicrographs because the mounting medium has an appropriate refraction index in relation to visible light even though air may obscure some details of the cuticle. The slide preparation must be done with great care, without exerting undue pressure, to avoid artifact.11
It is important to examine both the proximal and distal ends of the hair shaft because the more specific changes are usually found in the proximal segment. Polarized light microscopy must be used in cases of children with alopecia characterized by short and fragile hairs. This technique is particularly crucial in the differential diagnosis of trichothiodystrophy, as it shows the hair shafts with alternating light and dark transverse bands (tiger tail pattern). In loose anagen hair syndrome, special stains such as toluidine blue or elastic van Gieson must be performed to adequately visualize the internal root sheath.12
Hair bulb examination
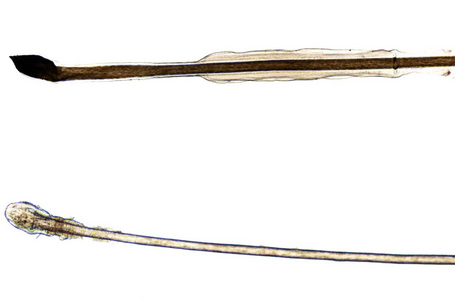
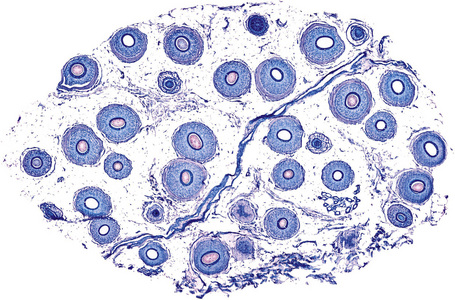
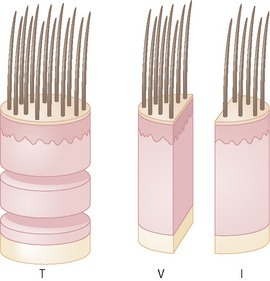
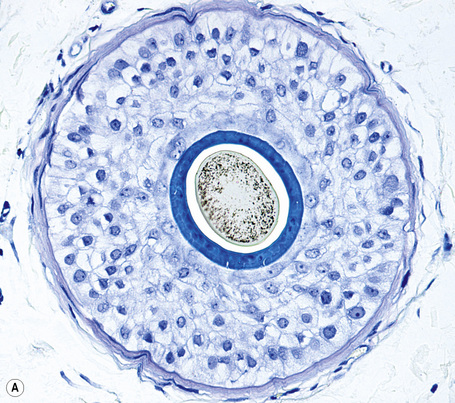
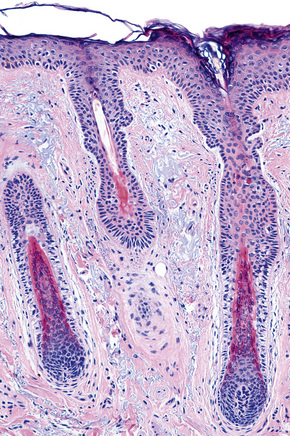
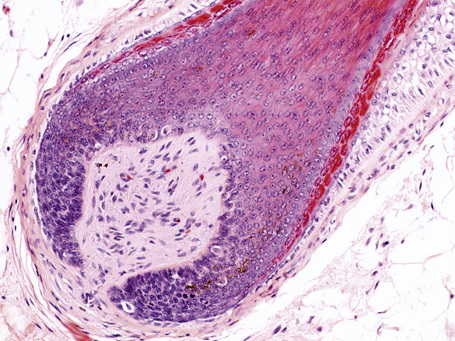
The hair bulbs present the following characteristics (Fig. 22.1):

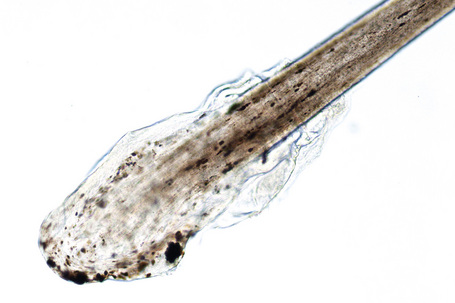
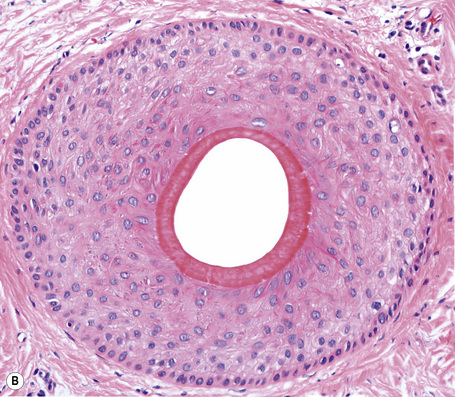
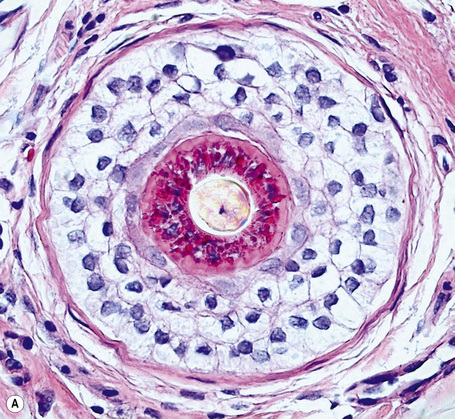
Fig. 22.2 Anagen hair: hair bulb detail. Note the difference in shape and pigmentation compared with the telogen hair bulb shown in Figure 22.3.
Trichoscan
The trichoscan may be considered as a noninvasive modification of the trichogram which combines epiluminescence microscopy with automatic digital image analysis for the measurement of human hair. It is able to analyze many biological parameters of hair growth (density, diameter, growth rate, vellus and terminal hair density). It can be used in clinical studies to compare the different efficiency of various hair growth-promoting substances.13,14
Scalp biopsy and biopsy report
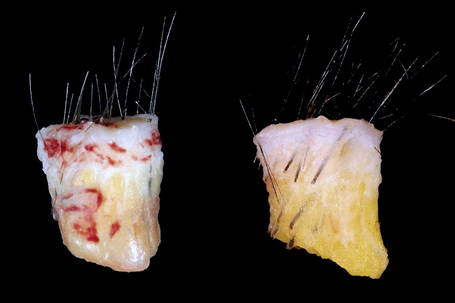
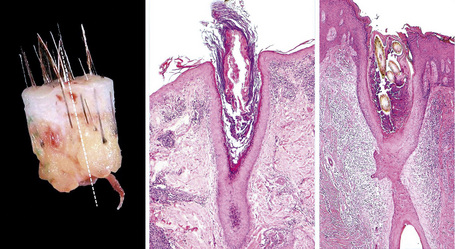
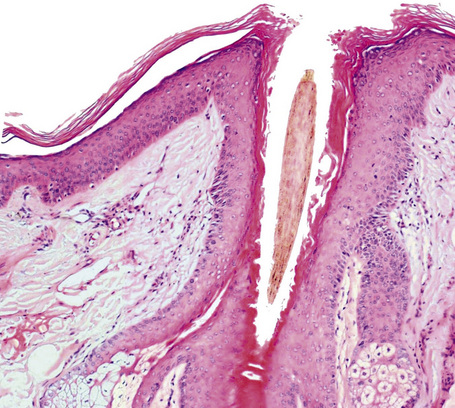
Scalp biopsy is a very valuable tool in the diagnosis of hair loss. Ideally, two deep punch biopsies 4 mm in diameter should be obtained for comparison from different sites: one should be taken from the affected area and the second from the periphery where there is healthy scalp. An alternative is to take the two biopsies from different involved areas (e.g., in cases of diffuse or scarring alopecia). Sometimes a single biopsy is enough to make an accurate diagnosis but this depends on the level of experience of the clinician and the pathologist. It is highly recommended to verify that the internal diameter of the punch which is to be used really measures 4 mm (0.125 cm2) as this measure varies from one company to another.1 The biopsy is taken following the direction of the hair follicles with the intention of minimizing tangential sections (Fig. 22.4). It should include epidermis, dermis, and a very generous amount of subcutaneous tissue. The obtained specimen must be delicately handled, avoiding any crushing by the forceps, and quickly placed in 10% neutral buffered formaldehyde.
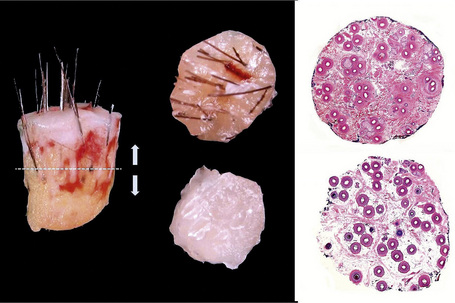
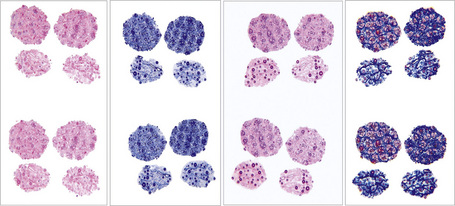
For the transverse sections the sample should be divided 2 mm away from the epidermis and serial sections obtained from both parts towards the surface and the deep layers of the specimen. In this way, the sections obtained from the upper portion will sequentially approach the surface epithelium and those derived from the lower portion will sequentially approach the deep dermis and subcutaneous fat (Fig. 22.5). Afterwards, the basic stainings are performed with hematoxylin and eosin and electively with toluidine blue, PAS, Masson’s trichrome, and elastic tissue stain (Figs. 22.6, 22.7). The best sections to evaluate terminal anagen hair follicles/vellus hair and hair follicles in anagen, catagen, and telogen are those obtained at the limit between the upper and lower segment of the hair follicle (at level of the bulge).
Although transverse sections of scalp skin have been studied for over 100 years, it wasn’t until 1984 that Headington carried out the first systematic studies of scalp biopsies using serial sections.2,3 This method represented a significant advance in the diagnosis and understanding of diseases of the hair. The Headington technique demonstrated that transverse sections of biopsies obtained with a 4-mm punch provided excellent histological material for morphometric and quantitative analysis, including the different phases of the normal hair growth cycle.4,5 This technique has been modified over the years and multiple horizontal sections throughout the sample are evaluated, enabling the pathologists to correctly evalute most types of alopecia including chronic telogen effluvium, male androgenetic alopecia, alopecia areata, and the various types of scarring alopecia, and to perform digital image studies in cases of nonscarring alopecias.6–13 It is also a very useful method to evaluate the results on the hairs of the administration of a given drug.14
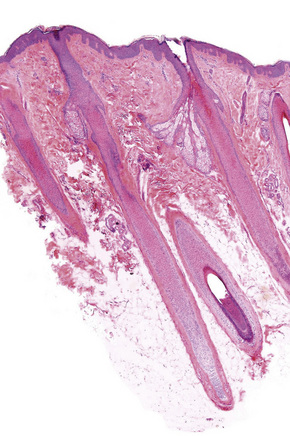
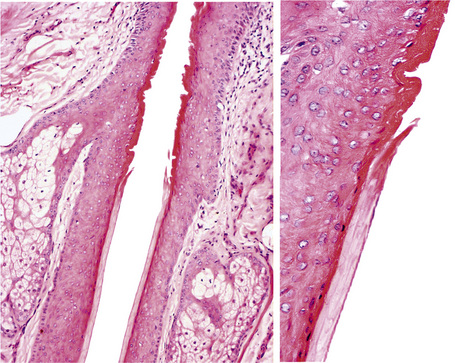
Vertical sections were exclusively used for many years and they provide a panoramic view from the epidermis to the hypodermis, more familiar to the general pathologist and to the dermatopathologist. These allow clear differentiation of all compartments of the skin, especially the dermoepidermal junction and superficial dermis. This is particularly useful in the diagnosis of scarring alopecias which involve the interface such as discoid lupus erythematosus and lichen planopilaris (Fig. 22.8). However, only very few hair follicles are visualized with vertical sections (at best 10–15% of the total in a single section) and often these are sectioned transversely or obliquely (Fig. 22.9).3 It is not an adequate technique for quantitative studies or for establishing the ratios between the different phases of the hair follicles (anagen/telogen ratio, terminal/vellus hair ratio), hence its use is very limited in the diagnosis of androgenic-type alopecias and those of chronic telogen effluvium. (Tables 22.1, 22.2) It is universally accepted that both techniques present obvious advantages and disadvantages and if two biopsies are available the best option is to use one for the transverse sections and the other for the vertical sections (Fig. 22.10), hence using the second half of the latter one for complementary studies (cultures, immunofluorescence, electron microscopy).15–17
Table 22.1 Advantages and disadvantages of vertical and horizontal sections for the diagnosis of the alopecias.
| Type of section | Advantages | Disadvantages |
|---|---|---|
| Vertical | Easy to perform and interpret Good representation of the dermoepidermal interface | Few hair follicles and majority in oblique section Difficult to standardize and not suited for quantitative studies |
| Transverse (horizontal) | Follicles are seen in only one plane Easy to standardize and useful for morphometric studies | Difficult to perform and interpret Poor representation of the dermoepidermal interface |
Table 22.2 Indications for vertical and transverse sections according to the type of alopecia
| Type of section | Indication |
|---|---|
| Transverse (horizontal) and/or vertical* | Primary and secondary scarring alopecias Alopecias that affect the dermoepidermal interface Alopecia areata Trichotillomania Infectious type alopecias (fungi, syphilis, herpes, etc) |
| Transverse (horizontal) | Morphometric studies that require quantitative analysis and comparative studies in diseases such as: androgenetic alopecia and chronic telogen effluvium Studies involving drugs |
* Transverse and horizontal biopsies may be both used if there is sufficient material available.
Hair biopsy report
It is important that all quantifiable data are included in the histology report. This often allows for an accurate diagnosis when the findings are correlated with the clinical information (Box 22.1).
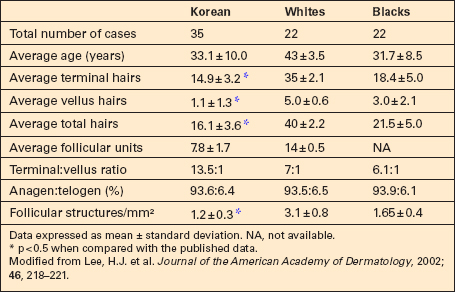
Even though the microscopic features, chemical composition, and molecular structure are very similar in different ethnic groups, significant differences in conformation, mechanical properties, and capacity to absorb water according to ethnic origin have been described.18–20 Especially important are the striking differences in hair density and relative proportion of hair follicles in anagen/telogen and terminal/vellus hairs in blacks, Asians, and Caucasians, which should be always taken into consideration when interpreting scalp biopsies (Table 22.3).21–24
Embryology and anatomy of the normal hair follicle
Embryology
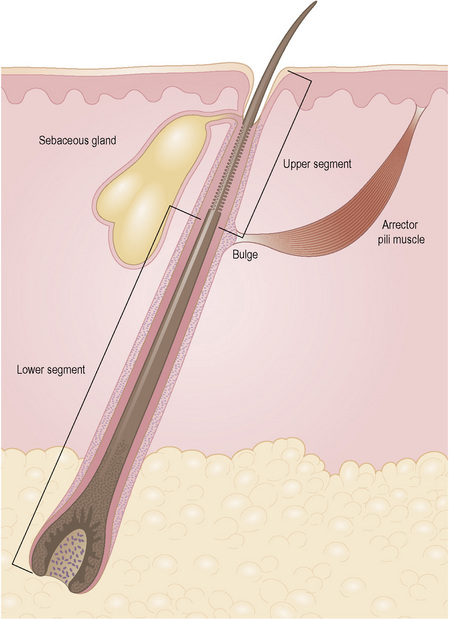
Embryologically, each follicle is composed of epithelial and mesenchymal components. Epithelial invaginations (placodes) derive from the fetal epidermis and project downwards at regularly spaced intervals, proliferating under the influence of the underlying connective tissue cells which form the mesenchymal condensation of the hair peg. This occurs around the tenth week of gestation (Fig. 22.11). This dermal condensate will eventually mature into the dermal papilla. The follicle therefore does not develop in the absence of this mesenchymal influence. Afterwards, the hair follicle elongates into a lace of epithelial cells and the deeper ones form the bulbous and the matrix cells, which give origin to the hair shaft and inner root sheath. The outer root sheath forms three bulges. The upper one will give origin, in the follicles located in the anogenital region, axillae, areolae, periumbilical region, eyelids, and external ear canals, to the apocrine glands. The middle bulge will give origin to the sebaceous gland. The lowermost bulge corresponds to the location of the epithelial stem cells. This is also the attachment place of the arrector pili muscle.1
The genes and molecules that participate in the development of the follicle have been extensively studied. Different positive and negative regulators which express themselves at variable moments of the follicles development have been identified.2,3 Some of the most significant are: beta-catenin important to enable the epidermis to form a hair follicle. Artificial activation of beta-catenin in the basal epidermic cells in adult transgenic mice has the ability to develop hair follicles.4 In relation to the latter, transgenic mice expressing an activated beta-catenin are predisposed to acquire skin tumors resembling pilomatricoma.5 Ectodysplasin (EDA) promotes the development of hair follicles. Mutation of the EDA gene causes X-linked anhidrotic ectodermal dysplasia characterized by sparse hair, abnormal or missing teeth, and an inability to sweat due to the lack of sweat glands.6 Gata-3, a key regulator of T-cell lineage determination, is expressed at the onset of epidermal stratification and internal root sheath specification in hair follicles.7 Finally, members of the bone morphogenetic family (BMP2 and BMP4) function as inhibitors of the follicle formation.8
Anatomy
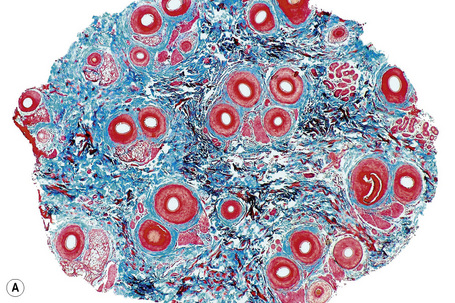
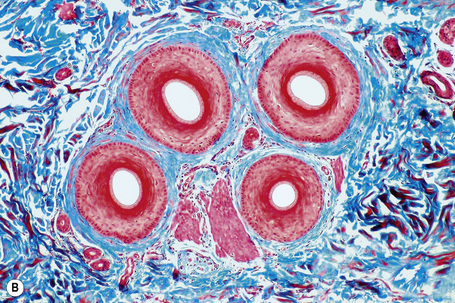
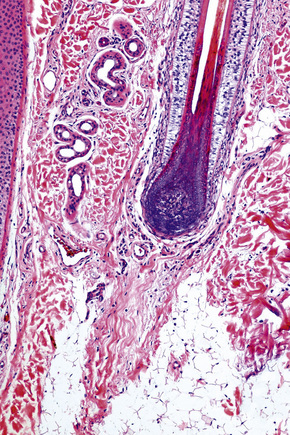
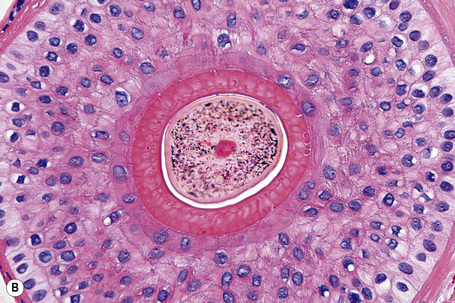
Horizontal sections of the upper segment of the hair follicle show that hair follicles in the scalp are grouped, forming anatomic structures known as follicular units, which are composed of terminal and vellus hairs, sebaceous glands, and arrector pili muscles. The distribution of hair follicles in follicular units is better appreciated at the level of the infundibulum and isthmus. Masson’s trichrome is a particularly useful stain to delineate the follicular unit because the connective tissue surrounding the outer root sheath tends to condense around discrete units comprising three to six hair follicles, one or two of which represent vellus hairs (Fig. 22.12).
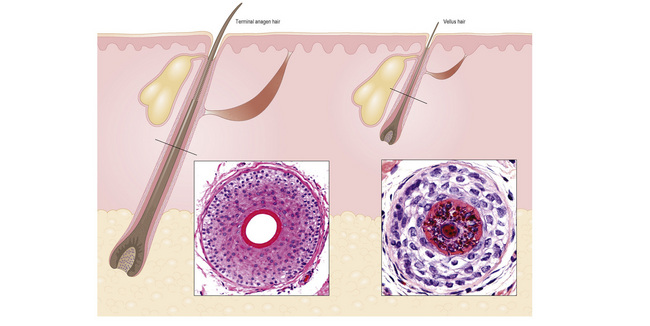
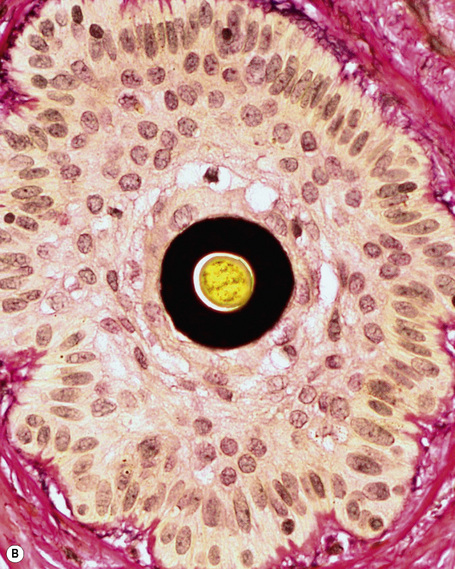
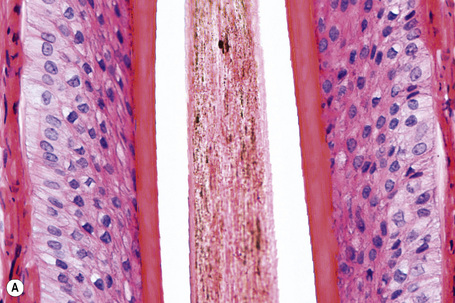
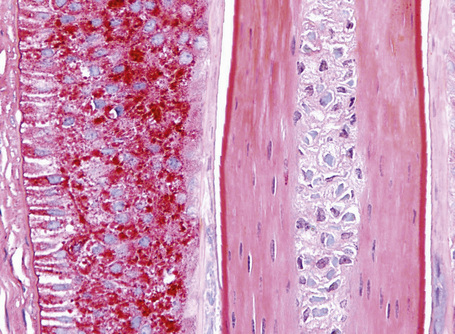
Terminal hair follicles are easy to recognize in horizontal sections because they are much larger than vellus hairs (Fig. 22.13). They are thick, pigmented, and usually descend to the subcutaneous fat (Fig. 22.14). They contain a hair shaft, the diameter of which is greater than the thickness of the inner root sheath, and generally measures in excess of 0.06 mm (Fig. 22.15).
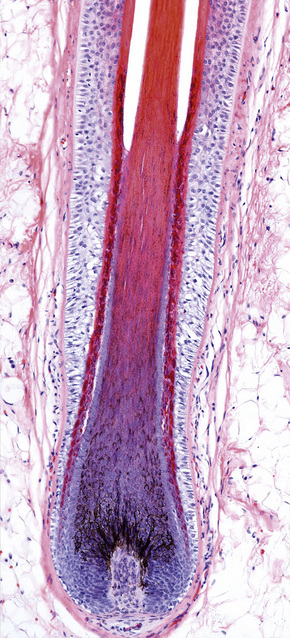
Fig. 22.14 Terminal anagen hair: the pigmented hair bulb is embedded deeply in the subcutaneous fat.
True vellus hairs are thin, short, and nonpigmented, and are characterized by a hair bulb that only extends to the upper or middle reticular dermis (Fig. 22.16). The arrector pili muscle is usually undetected. The hair shaft diameter is equal to or less than the thickness of the inner root sheath (Fig. 22.17). It is typically less than 0.03 mm.
Vellus-like hairs (miniaturized hairs) are very similar to vellus hairs. They correspond to terminal hair follicles that have miniaturized due to the effect of androgens. They may be very difficult to differentiate from true vellus, except for the fact that the miniaturized follicles leave a stellate trail of collapsed fibrous tissue, the follicular stellae (Fig. 22.18). In a biopsy of scalp, when reference is made to vellus in general without qualifying its origin, it refers by convention to miniaturized hair follicles and also to true vellus hairs.
The hair shaft and the inner root sheath are easily identified in a vertical and horizontal section at the level of the stem with conventional staining procedures including hematoxylin and eosin, toluidine blue, and elastic stains (Fig. 22.19). The ratio between terminal and vellus hairs is approximately 7:1. Distinguishing between terminal and vellus hair follicles is critical for the diagnosis androgenetic and temporal triangular alopecia. A ratio of 4:1 or less is suggestive of androgenetic alopecia. It is important to bear in mind that the hair shaft is frequently lost during the biopsy process. In these cases its size may be extrapolated from the diameter of the empty space delimited by the inner root sheath.
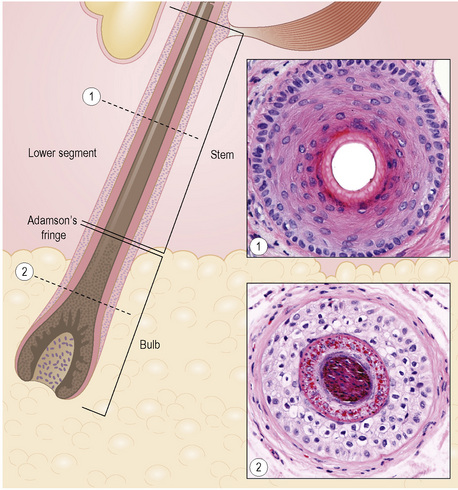
The hair follicle can be anatomically and functionally divided into two distinct segments (Fig. 22.20). Differences between these two elements are explained by the fact that the upper portion of the hair follicle is very stable and not affected by maturation and shedding of the hair. The lower portion of the hair follicle is actively involved in hair growth and undergoes considerable morphological change according to its stage in the hair cycle.
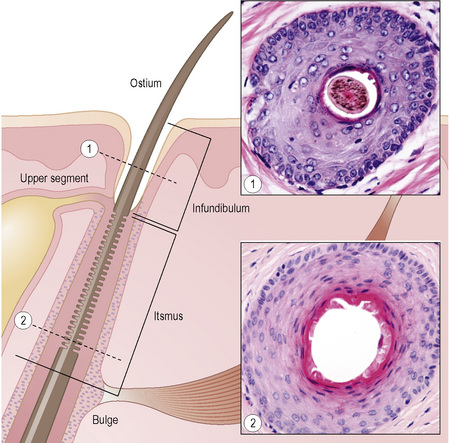
The upper segment of the hair follicle consists of the follicular ostium, the infundibulum, and the isthmus (Fig. 22.21). The infundibulum extends from the ostium downwards to the opening of the sebaceous gland duct (Fig. 22.22). The isthmus continues from the opening of the sebaceous gland duct to the site of attachment of the arrector pili muscle at the hair bulge (Fig. 22.23). The arrector pili muscle attaches to the bulge area through elastic tendons and extends to its upper attachment in the adjacent epidermis.
The lower segment extends from the arrector pili insertion to the lower part of the hair bulb and has two components: the stem that extends from the arrector pili insertion to the Adamson’s fringe and the lower portion called the bulb (Figs 22.24, 22.25). This comprises the matrix cells and the basal melanocytes that surround the dermal papilla (Fig. 22.26).9–11
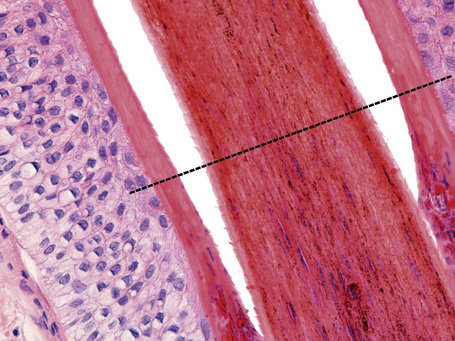
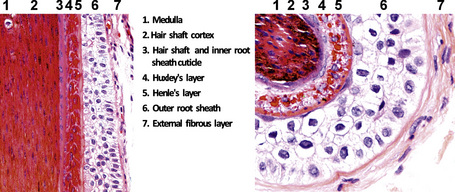
A hair shaft is composed of three layers: the medulla, the cortex, and the cuticle. The medulla, which represents the central region of the hair shaft, is not consistently present in humans but it is often an important component of hair in other animals (Figs 22.27, 22.28). The cortex is the thickest layer and is responsible for the strength of the shaft. It is composed of intermediate filaments of hard keratin that are arranged in microfibrils, which intertwine to form cable-like structures called macrofibrils. The cuticle, which constitutes the outer part of the shaft, is responsible for the resistance to the wear and tear produced by the environment. It consists of scales orientated at right angles to the epidermis. These interlock with the cuticle of the internal root sheath maintaining integrity (Fig 22. 29). The linear or curved shape of the hair is determined by the curved or linear shape of the internal radicular sheath. In transversal sections curved hairs are elliptical and linear hairs are circular.
These layers change noticeably depending on the level of the microscopic section. The cuticle of the hair shaft and all layers of the internal root sheath at bulb level undergo trichohyaline keratinization. At the bulge, the inner root sheath disappears and is replaced with trichilemmal keratin derived from the external root sheath (Fig. 22.23).12
The external root sheath is continuous with the epidermis at the infundibulum and there forms a granular layer and basket weave epidermal keratin (Fig. 22.22). Towards the bulb, the cells of the outer root sheath cells contain abundant glycogen (Fig. 22.29).
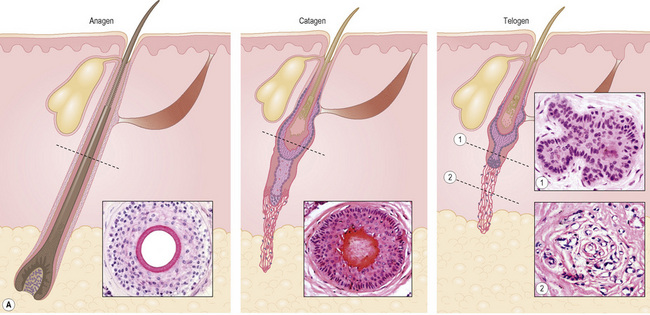
Hair cycle
The hair growth cycle, whether terminal or vellus, is divided into three stages: active growth (anagen), involution (catagen), a period of rest (telogen) and, as an extension of this last stage, two additional stages: hair shaft-extrusion (exogen) and empty hair follicle (kenogen) (Fig. 22.31). Human beings differ from other mammals in that these stages occur continuously rather than synchronously. There is no periodic change of the hair covering the body.
The interaction of numerous growth factors, cytokines, hormones, neurotransmitters, and their receptors are important in the development and cycling of normal hair follicles. It appears that the driving force of cycling, the ‘hair cycle clock’, is located in the hair follicle itself and some neurotrophins, of the brain-derived neurotrophic factor type, are important in the induction of the catagen phase. However, no single growth factor appears to exert ultimate control over these processes.1–3
Anagen lasts from 2 to 7 years and is characterized by continuous growth, giving place to a long and pigmented pilar stem, which is easily visible. It is the phase that determines the length of the hair and the phase that varies the most depending on the body location. Morphologically, it corresponds to the already mentioned terminal hair follicles deeply situated in the subcutaneous fat (see Figs 22.14, 22.20). The pilar bulb of the follicles in anagen shows abundant melanin production and intense mitotic activity, resulting in the hair growing approximately 1.0 cm per month. At any particular moment in time between 80% and 100% of the hair follicles are in anagen (Fig 22.31). As this is the phase with the highest mitotic activity, melanogenesis, and DNA synthesis, it is the most vulnerable to hormonal changes, drugs, and different toxins.
Pigmentation of the hair only occurs in anagen and it is induced by interactions between bulbar melanocytes, keratinocytes, and dermal papilla fibroblasts (hair follicle pigmentary unit) (Fig. 22.32).4 The coupling of hair follicle melanogenesis to the anagen phase distinguishes follicular melanogenesis from the continuous melanogenesis of the epidermis. Cyclic reconstruction of the hair follicle pigmentary unit occurs optimally during only the first 10 hair cycles, i.e., until approximately 40 years of age.5 It is not clear if hair graying is a consequence of functional loss, or a selective melanocyte depletion of human hair.6
Catagen precedes telogen and during this phase the inferior segment of the follicle undergoes massive apoptosis, reducing its size. It is the shortest phase and lasts from 2 to 3 weeks. Only 1% to 2% of the follicles are in catagen and it is rare to find them in normal scalp biopsies. Horizontal sections are characterized by diffuse apoptosis and an absence of mitotic and pigmentary activity. Apoptotic cells have intensely eosinophilic cytoplasm and a dark pyknotic nucleus (Figs 22.33, 22.34). As the follicle retracts, the vitreous membrane collapses and appears thickened and corrugated (Figs 22.35, 22.36). The inner root sheath disappears and the outer root sheath appears morphologically similar to the epithelium of the isthmus, surrounding a club-shaped hair shaft and keratinizing in trichilemmal manner. Catagen follicles are frequent in trichotillomania.
< div class='tao-gold-member'>
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree