Chapter 7 Lichenoid and interface dermatitis
The term ‘lichenoid’ refers to inflammatory dermatoses which are characterized by a bandlike lymphohistiocytic infiltrate in the upper dermis, hugging and often obscuring the dermoepidermal interface. Lichen planus is the prototypic lichenoid dermatitis (Box 7.1). Interface dermatitis refers to the presence of basal cell vacuolization (hydropic degeneration) and is often accompanied by single-cell keratinocyte apoptosis (Box 7.2). These two terms are by no means mutually exclusive as most lichenoid infiltrates are accompanied by interface change. However, some dermatoses are characterized primarily by interface change without a lichenoid infiltrate such as lupus erythematosus and erythema multiforme.
Box 7.2 Causes of interface dermatitis
Lichenoid dermatoses
Lichen planus
Clinical features
Lichen planus (Gr. leichen, tree moss) is a common, usually intensely pruritic, symmetrical, papulosquamous dermatosis.1,2 Its prevalence in the general population is approximately 1%, and it most often presents in the fourth to sixth decades with a slight female predominance.3,4 It is uncommon in childhood.5,6 Occasional familial cases have been reported.7,8
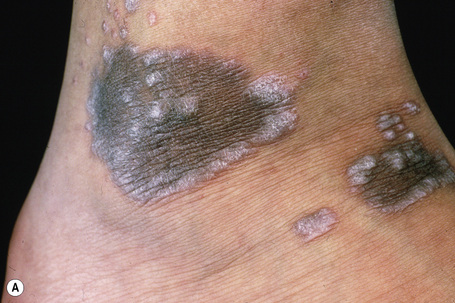
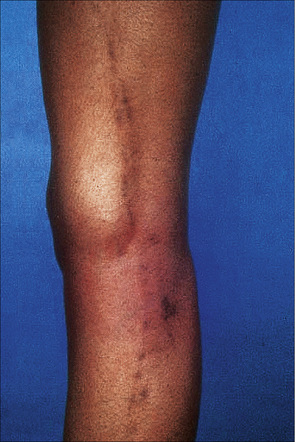
The disease is characterized by small, smooth, shiny, flat-topped polygonal papules measuring several millimeters to 1 cm in diameter and often having a violaceous color (Fig. 7.1). Delicate white lines known as Wickham’s striae typically cross the slightly scaly surface (Fig. 7.2). The lesions are found most commonly on the flexor aspect of the wrists, the forearms, the extensor aspect of the hands and ankles, the lumbar area and the glans penis (Fig 7.3). Lichen planus is associated with a positive Koebner’s phenomenon. It is a usually self-limiting although sometimes protracted disorder, patients clearing of lesions within weeks to 1 or 2 years.
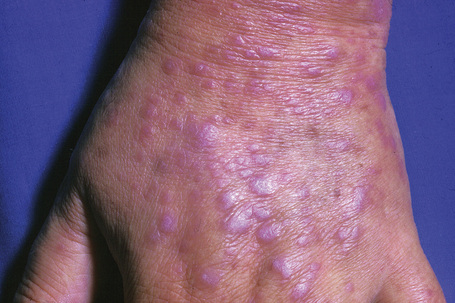
Fig. 7.1 Lichen planus: there are typical flat-topped polygonal papules on dorsum of the hand.
From the collection of the late N.P. Smith, MD, the Institute of Dermatology, London, UK.
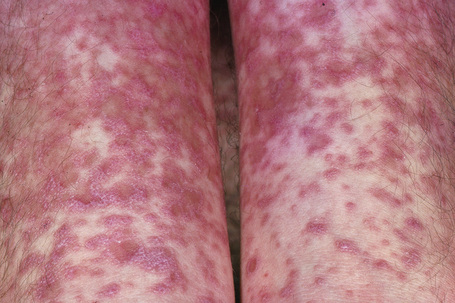
Fig. 7.3 Lichen planus: there is extensive bilateral involvement of the flexor aspect of the forearms.
From the collection of the late N.P. Smith, MD, the Institute of Dermatology, London, UK.
Oral involvement, which is very common (affecting up to 60% of patients with cutaneous disease), shows a marked female preponderance and presents most often in the seventh decade. It may sometimes be the sole manifestation (an estimated 15–35% of patients with oral lichen planus never develop skin lesions).9–14 The buccal mucosa, vestibule, tongue, and gingivae are most often affected, in decreasing order of frequency.12 Patients frequently present with a white lacelike pattern, but papules, plaques and erosions, ulcerated, atrophic, and bullous variants may also be found (Figs 7.4–7.6).1,15 Lesions are usually asymptomatic, although erosions and bullae are sometimes tender and painful. Chronic ulcerated oral lichen planus is of particular importance because it has been related to an increased risk, albeit low, of developing squamous cell carcinoma (Fig 7.7). The risk of developing malignancy is debated, with current literature suggesting that 0–12.5% of affected patients will develop an oral malignancy.12,16–22 Oral involvement in lichen planus and its relationship to cutaneous squamous cell carcinoma is discussed in greater depth elsewhere. Involvement of the gums may present as desquamative gingivitis.1 Other mucous membranes that may be involved include those of the pharynx, larynx, esophagus, nose, anus, and genitalia.23 Familial cases of lichen planus limited to oral involvement are noted.24
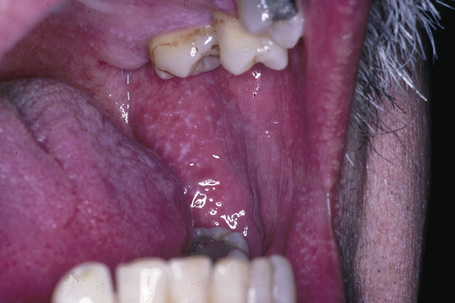
Fig. 7.4 Lichen planus: this lace-like pattern is characteristic.
From the collection of the late N.P. Smith, MD, the Institute of Dermatology, London, UK.
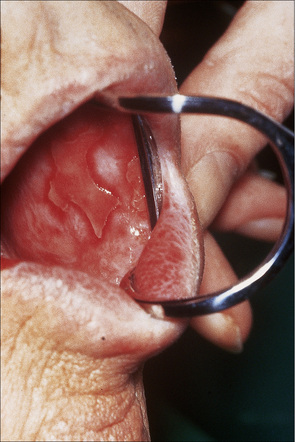
Fig. 7.5 Lichen planus: there is extensive ulceration of the buccal mucosa.
By courtesy of R.A. Marsden, MD, St George’s Hospital, London, UK.
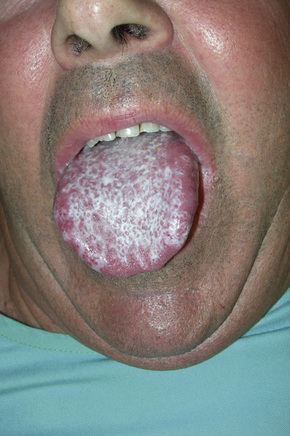
Fig 7.6 Lichen planus: the tongue is commonly affected.
By courtesy of M. Blanes, MD, Alicante, Spain
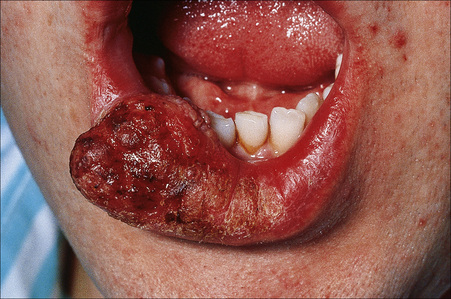
Fig. 7.7 Lichen planus: there is an ulcerated squamous carcinoma on the lower lip.
By courtesy of R.A. Marsden, MD, St George’s Hospital, London, UK.
Ocular involvement is rare and may include eyelid lesions, blepharitis, conjunctivitis, keratitis, punctate corneal opacities, iridocyclitis, and chorioretinitis.25,26
Esophageal involvement, although rare, is an important potential cause of morbidity, and is the most frequently involved gastrointestinal site.27 Concomitant oral lesions are invariably present. To date, middle-aged or elderly females are typically affected.28–31 Complications include chronic dysphagia and stricture formation affecting the mid or upper esophagus.28,32–35 Patients with esophageal lichen planus may have a risk of developing squamous cell carcinoma. The role of surveillance is uncertain.27,28,30,31,36,37
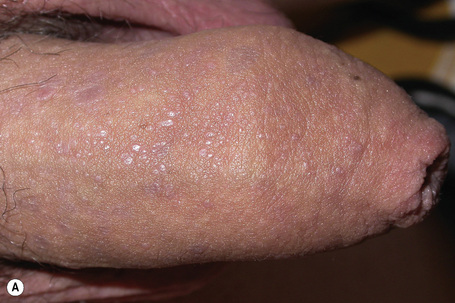
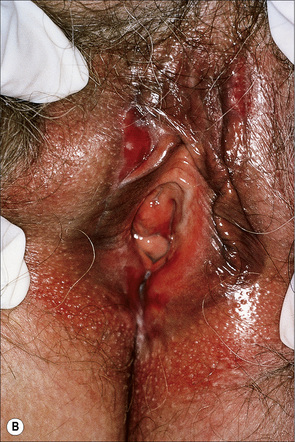
Genital lesions in lichen planus are common (particularly in males), being present in up to 25% of patients, and sometimes adopting an annular configuration (Fig. 7.8).1 Similar annular lichen planus may be found elsewhere on the body, including intertriginous areas.38 Occasionally, penile lesions are the sole expression of the disease.39 Vulval lesions may be found in up to 51% of females with cutaneous involvement.40 Sometimes gingival and female genital lesions may coexist as a variant of erosive lichen planus, the so-called vulvovaginal-gingival syndrome.41–44 Patients present with dyspareunia and intense burning vulval pain. The vulva appears congested and there may be erosions, which are often surrounded by a white reticulate border. Vaginal involvement similarly presents as dyspareunia and often postcoital bleeding due to inflammatory, desquamative, and erosive changes. More typical features of lichen planus may be encountered elsewhere on the body. Squamous carcinoma is an important complication of chronic vulval lichen planus.45 The development of penile cancer is rare.46 Genital involvement in lichen planus is discussed elsewhere.
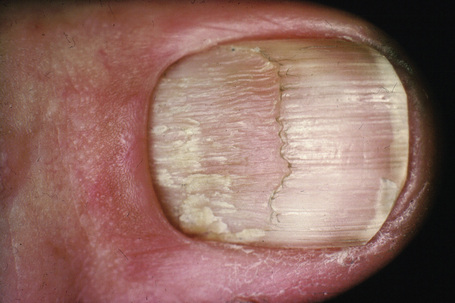
The nails are affected in about 10% of patients with lichen planus; manifestations include thinning of the nail plate, longitudinal ridging, striations, pterygium formation, subungual hyperkeratosis and, very rarely, complete destruction of the nail (Figs 7.9).1 Although nail involvement in children is said to be rare, some authors regard twenty-nail dystrophy of childhood as a variant of localized lichen planus, although not all accept this hypothesis.47–51
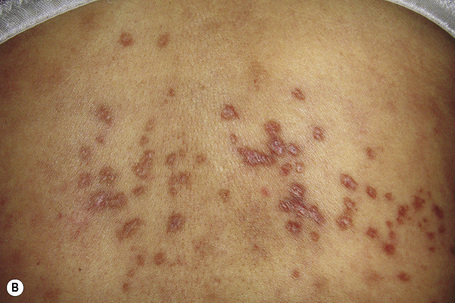
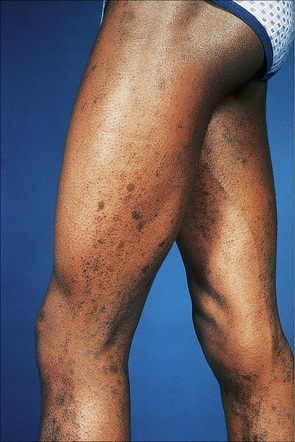
Most lesions of lichen planus heal within 6–18 months of onset. However, oral and hypertrophic variants and lichen planopilaris tend to have a chronic course. Postinflammatory hyperpigmentation, which may be very disfiguring, is not uncommon, particularly in colored races (Fig. 7.10).
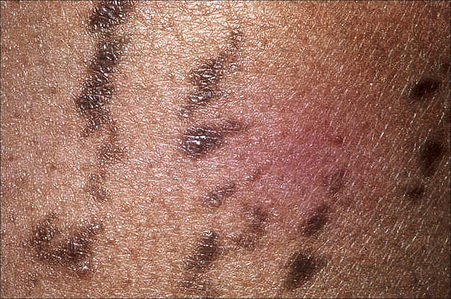
Fig. 7.10 Lichen planus: postinflammatory hyperpigmentation is a common manifestation.
By courtesy of R.A. Marsden, MD, St George’s Hospital, London, UK.
A number of variants of lichen planus merit specific mention:
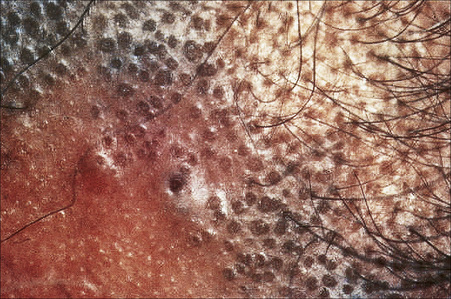
Fig. 7.13 Lichen planopilaris: follicular lichenoid papules are clearly seen in this patient.
By courtesy of the Institute of Dermatology, London, UK.
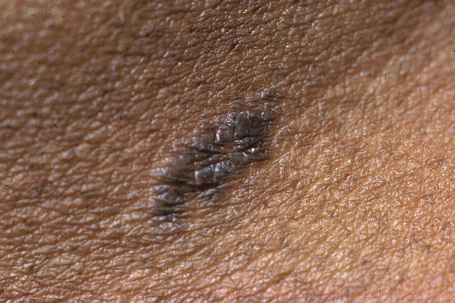
Fig. 7.15 Lichen planus pigmentosus: there are coalescent pigmented papules.
From the collection of the late N.P. Smith, MD, the Institute of Dermatology, London, UK.
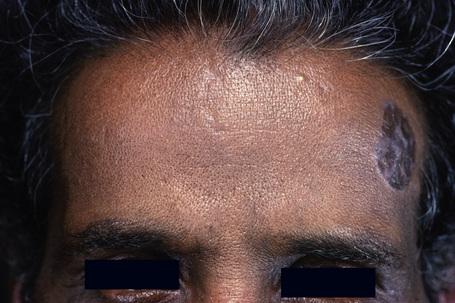
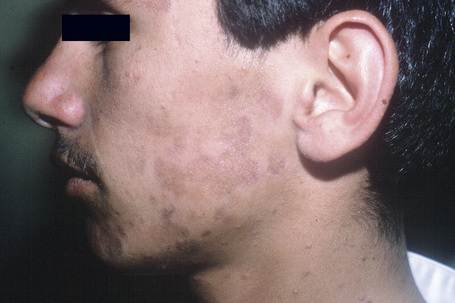
Fig 7.16 Lichen planus pigmentosus: the face is a commonly affected site.
From the collection of the late N.P. Smith, MD, the Institute of Dermatology, London, UK.
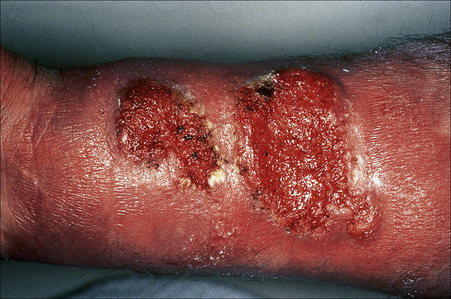
Fig. 7.18 Ulcerative lichen planus: there is marked atrophy of the skin around this crusted ulcer.
By courtesy of the Institute of Dermatology, London, UK.
Other variants include lichen planus linearis, which occurs predominantly in children, and the rare vesicular or bullous variants, which must be distinguished from lichen planus pemphigoides. Bullous lichen planus implies the development of vesicles or bullae on pre-existent lichenoid lesions as a consequence of severe basal cell hydropic degeneration. It is more often a histological finding rather than a clinical observation. In contrast, lichen planus pemphigoides is characterized by the development of large tense bullae arising on normal or erythematous skin in a patient with typical lichen planus elsewhere. It represents the combined expression of lichen planus and bullous pemphigoid.77
Childhood lichen planus shows a modest male predominance (2:1).5,6,61,78 Although mucosal involvement is said to be rare, recent series report a frequency of 14–39%.6,61,78 Hypertrophic lesions may be seen in up to 26% of cases.6
Pathogenesis and histological features
The etiology of lichen planus is unknown. Theories of infectious (bacterial and viral), autoimmune, metabolic, psychosomatic, and genetic causes have all had their proponents. Currently, however, it is thought that lichen planus represents an abnormal delayed hypersensitivity reaction to an as yet undetermined epidermal neoantigen, possibly to a combination of an external antigen coupled with an internal self-antigen.79,80 The association of lichen planus with a number of viral infections including hepatitis B and C and human immunodeficiency virus (HIV), combined with the well-recognized relationship to numerous drugs, adds support to this hypothesis.81–83
Lichen planus is associated with a variety of liver cell abnormalities including aberrant liver function tests and serology.84,85 An increased incidence of chronic active hepatitis, primary sclerosing cholangitis and primary biliary cirrhosis has also been recorded.86–91 Not all documented series, however, have confirmed these observations, suggesting that the reported relationship may be dependent upon the background level of hepatitis B virus infection.89 Lichen planus has also followed hepatitis B vaccination.92–95 More recently, lichen planus (particularly oral disease) has been linked to hepatitis C virus and chronic liver disease. The incidence of hepatitis C virus in patients with lichen planus is, however, very variable, ranging from effectively zero in some populations, including the United Kingdom, India, and Slovenia, to as high as 100% in Japan.96–101
Evidence of other disorders including thyroid disease, dyslipidemia, and impaired carbohydrate metabolism including overt diabetes mellitus has also been documented in lichen planus, particularly the oral variant.102–110 A recent study from Japan suggests a possible association of hepatitis C infection with both diabetes and lichen planus.109
A significant association between lichen planus and human leukocyte antigen (HLA)-DR1 and HLA-DQ1 has been noted by a number of authors.111–117 This association pertains to patients with or without mucosal lesions but does not extend to patients with the drug-induced variant. It is suggested that this association relates to antigen presentation by HLA-DR1+ cells to T-helper cells with the resultant development of an autoimmune response.111
Although it is generally accepted that the pathogenesis of the basal cell damage in lichen planus primarily involves the cellular immune response, the precise mechanism(s) require further elucidation. It is unlikely that autoantibody and immune complex-mediated damage have a significant role in the lichenoid tissue reaction.79,80
The initial event in the evolution of the lichen planus papule is destruction of the basal epidermal layer (keratinocytes and melanocytes).118,119 In the earliest stage of development, increased numbers of Langerhans cells are present within the epidermis and it is believed that these cells process modified epidermal antigens for presentation to T lymphocytes.120 Keratinocytes express HLA-DR and this is likely to be of pathogenetic importance. Subsequent migration with resultant CD8+ T-cell activation results in basal keratinocyte death due to the combined effects of interferon-gamma (IFN-γ), interleukin (IL)-6, granulocyte-macrophage colony stimulating factor (GM-CSF), and tumor necrosis factor alpha (TNF-α).81–83,121 The expression of FasR/FasL by the basal keratinocytes suggests that apoptosis is an important mode of cell death in lichen planus.122 The dermal infiltrate consists predominantly of Ia+, CD4+ lymphocytes.120,123 CD8+ lymphocytes are also present in close apposition to the dermoepidermal junction adjacent to foci of basal keratinocyte necrosis and are said to predominate in early lesions.121,123–125 B lymphocytes are scarce and plasma cells are characteristically absent in cutaneous lesions, except in the hypertrophic variant.
Development of the typical papule appears to be due to a combination of continued keratinocyte destruction and regenerative activity, with the latter depending upon the migration of epithelium from the edge of the lesion and from adjacent eccrine ducts, rather than from increased mitotic activity. There is little uptake of tritiated thymidine at the site of basal cell damage, but conspicuous uptake at the edges of the lesion and, as a reflection of regeneration, keratin 17 expression is also up-regulated in the suprabasal epithelium.126 The typical features of lichen planus therefore depend upon a variable interplay between basal cell liquefactive degeneration and irregular epidermal regeneration.
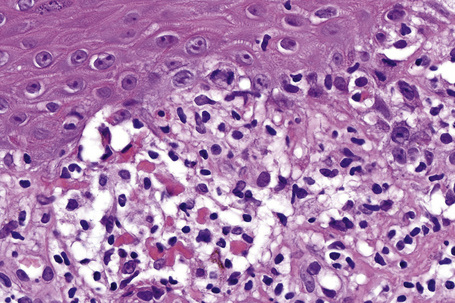
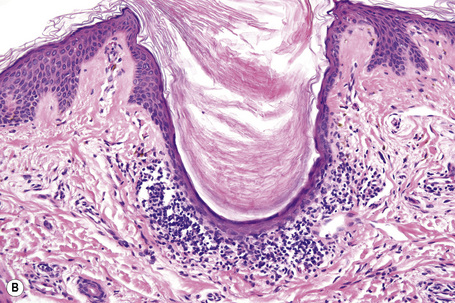
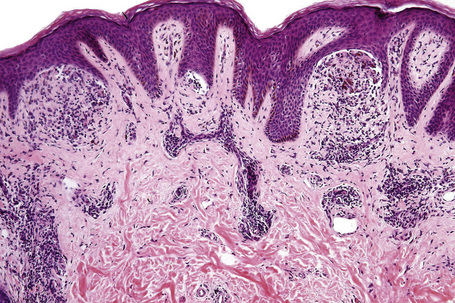
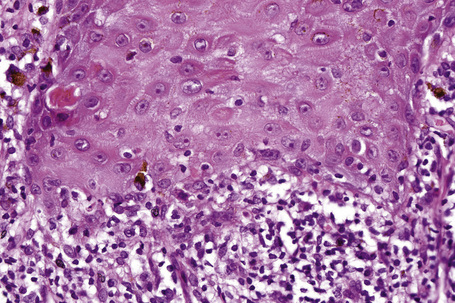
The earliest identifiable change in lichen planus is the presence of cytoid bodies and associated pigmentary incontinence. Cytoid bodies (colloid or Civatte bodies) are round or oval, homogeneous, eosinophilic bodies identifiable within the basal epithelium and the papillary dermis (Fig. 7.20). They display diastase-resistant periodic acid-Schiff (PAS) positivity, and may be identified within papules, perilesional skin, and even apparently uninvolved skin. Although they may be seen in a variety of dermatoses (including lupus erythematosus, graft-versus-host disease, and poikiloderma) and seemingly normal skin, where their presence, if either in large numbers or in a cluster, suggests lichen planus.
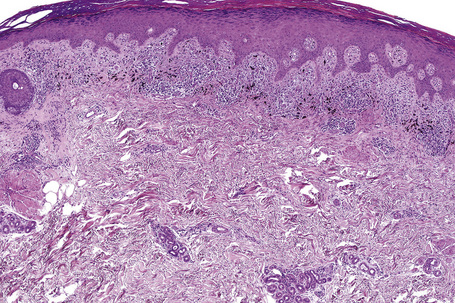
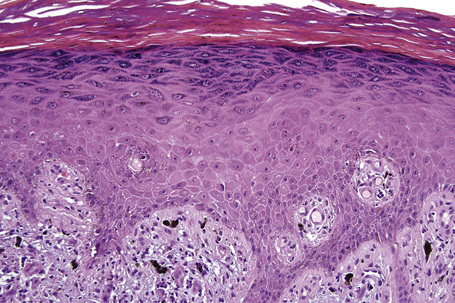
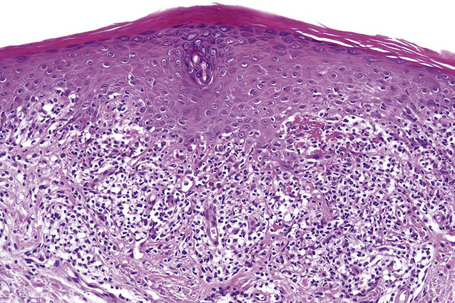
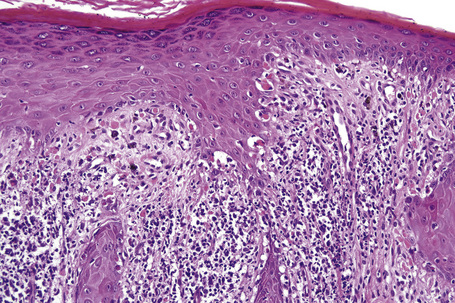
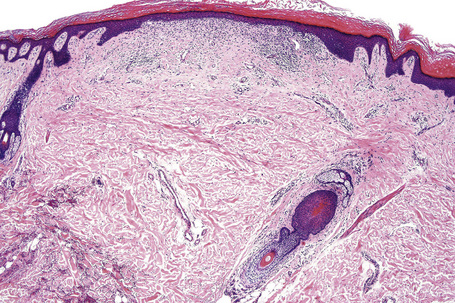
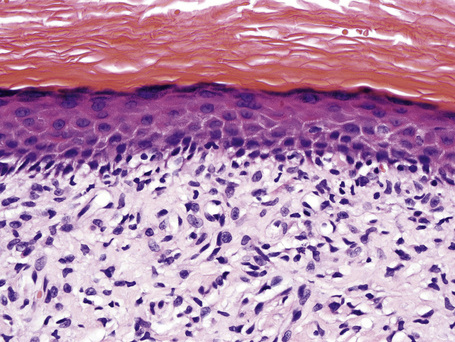
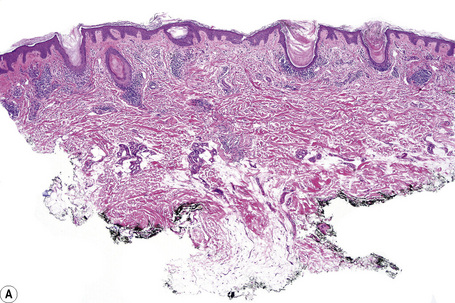
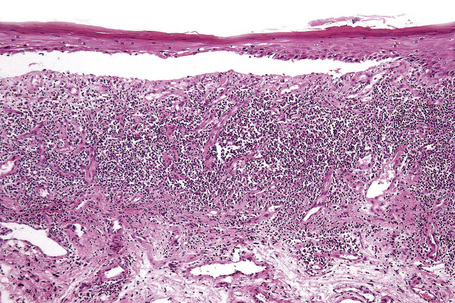
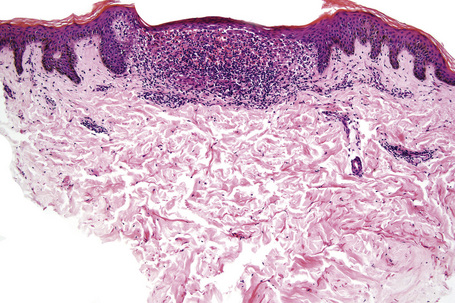
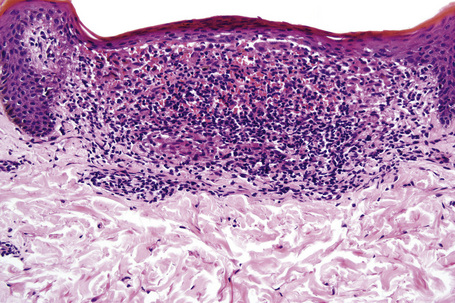
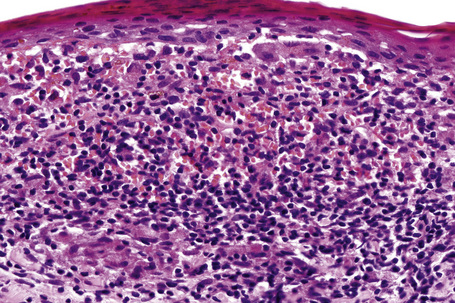
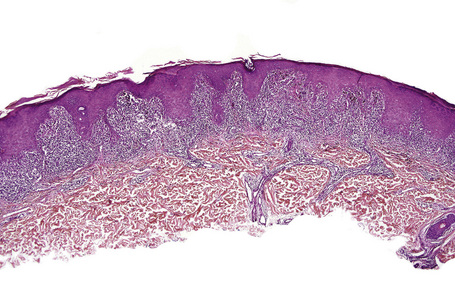
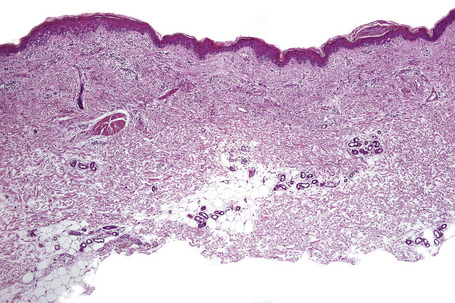
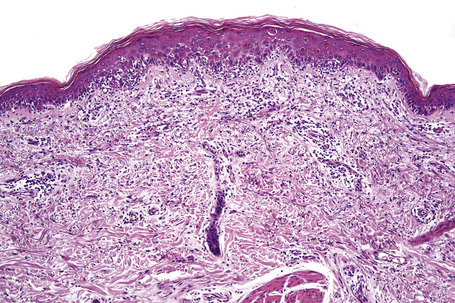
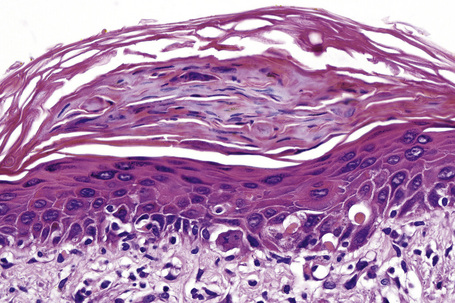
Characteristic histological features of an established papule can usually be recognized at scanning magnification (Fig. 7.21). They comprise hyperkeratosis, typically wedge-shaped hypergranulosis (clinically presenting as Wickham’s striae) related to the intraepidermal components of sweat ducts and hair follicles, and irregular acanthosis (Figs 7.22, 7.23). The acanthosis often has a saw-toothed appearance (Figs 7.24, 7.25). The presence of prominent parakeratosis argues strongly against a diagnosis of lichen planus. Lymphocytes and histiocytes may sometimes be seen in the epidermis and very occasionally satellite cell necrosis is a feature. Liquefactive degeneration of the basal layer of the epithelium is characteristic and often subepidermal clefts are present (Max Joseph spaces). Pigmentary incontinence is common (Fig. 7.26). A lymphohistiocytic bandlike infiltrate occupies the upper dermis and obscures the dermoepidermal junction. Hyperkeratosis persists in resolving lichen planus, but the acanthosis regresses, leaving a flattened epidermis (Fig. 7.27); there may be focal scarring and the dermal infiltrate is less conspicuous (Fig. 7.28).
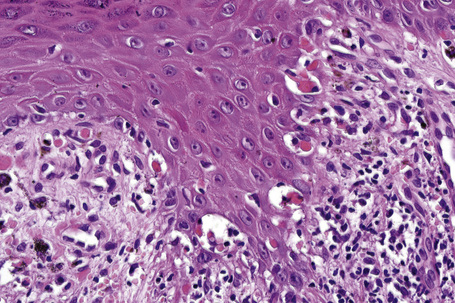
Fig. 7.25 Lichen planus: close-up view of Figure 7.24 showing basal cell liquefactive degeneration and cytoid bodies.
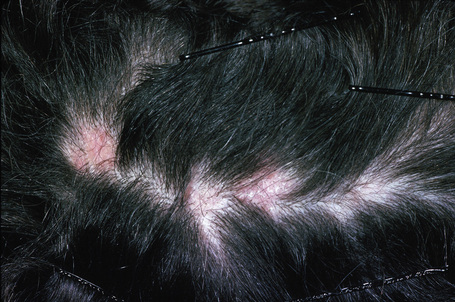
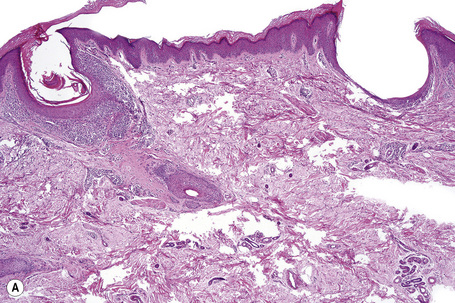
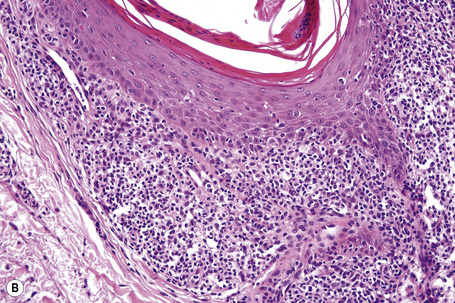
Lichen planopilaris in its early stages shows an infiltrate surrounding the lower hair follicle and papilla, follicular dilatation, and keratin plugging (Fig. 7.29).52,54 The adjacent interfollicular epithelium may or may not show a typical lichenoid infiltrate (Fig. 7.30). Basal cell hydropic degeneration, cytoid body formation, and pigmentary incontinence are also sometimes evident. In advanced scalp lesions, the hair follicles are destroyed and replaced by vertically orientated fibrous scars, reminiscent of the fibrous streamers seen in pseudopélade of Brocq.
Lichen planoporitis represents a rare variant in which lichenoid/interface changes are centered on the acrosyringium and eccrine sweat duct as it enters the epidermis. Squamous metaplasia of the ductal lining epithelium may be a feature.127
Lichen planus pigmentosus is characterized by epidermal thinning accompanied by basal cell vacuolization, pigmentary incontinence, and a superficial dermal lichenoid lymphohistiocytic infiltrate.4
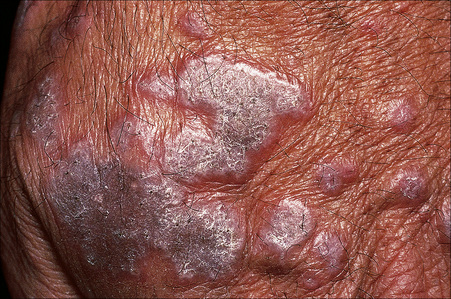
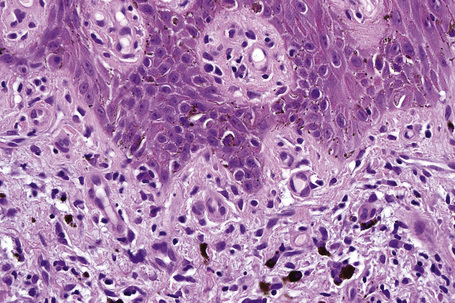
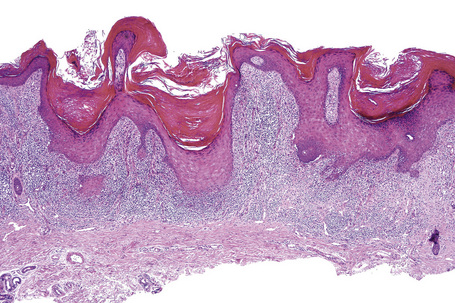
Hypertrophic lichen planus is characterized by more marked hyperkeratosis and acanthosis, with the epithelium sometimes showing pseudoepitheliomatous hyperplasia such that misdiagnosis as squamous cell carcinoma is a distinct possibility, particularly if clinical information is not available (Figs 7.31–7.33).73 A number of changes not seen in ordinary lichen planus may be observed and include parakeratosis, spongiosis, necrotic keratinocytes above the basal cell layer, and eosinophils and plasma cells in the dermal infiltrate. These changes may raise the possibility of a lichenoid drug eruption. The differential diagnosis is not difficult, as lichenoid drug eruptions tend to be more generalized and are not usually associated with hypertrophic changes.
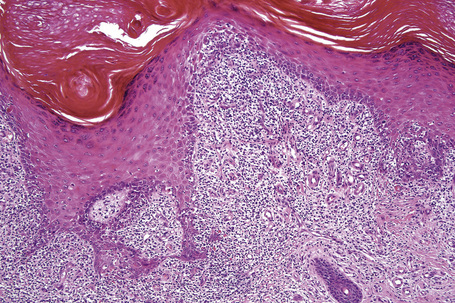
Fig. 7.32 Hypertrophic lichen planus: there is very marked irregular acanthosis. Note the hypergranulosis.
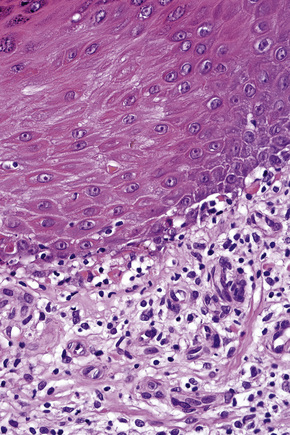
Fig. 7.33 Hypertrophic lichen planus: there is basal cell liquefactive degeneration with cytoid bodies.
Unlike skin involvement, esophageal lesions show parakeratosis. Variable epithelial atrophy and/or mild thickening are usually seen and the saw-toothed pattern of acanthosis is not a feature.28,32–34 As with oral lesions, plasma cells often accompany the lymphocytic infiltrate.
Vesicular or bullous lesions are subepidermal and occur due to excessive edema developing in association with the basement membrane zone damage complicating basal cell hydropic degeneration (Fig. 7.34).
Direct immunofluorescence studies on skin biopsies from patients with lichen planus usually reveal a linear fibrillar band of fibrin at the dermoepidermal junction (Fig. 7.35). The cytoid bodies may be highlighted non-specifically by the use of antisera, mainly to IgM, but also to IgG, IgA and C3 (Fig. 7.36). A lichen planus ‘specific antigen’, which is present in the prickle cell and granular cell layers, has been demonstrated by indirect immunofluorescence of patients’ serum with fetal skin.128 Whether this is of pathogenetic significance is unknown. Direct immunofluorescence of lichen planopilaris reveals follicular, linear basement membrane zone labeling with immunoglobulin (primarily IgG or IgA).129 Fibrin may also be present. The nosological implications of this observation are uncertain. Indirect immunofluorescence for circulating antibasement membrane zone antibodies is negative.
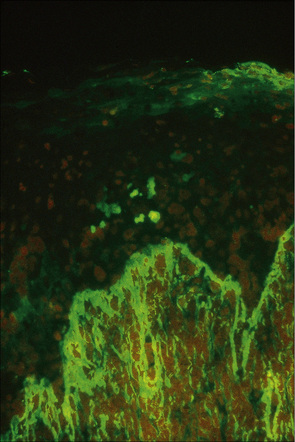
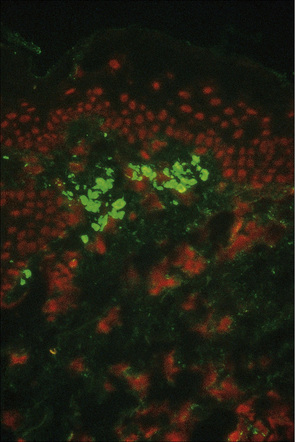
Fig. 7.35 Lichen planus: brilliant green fluorescence indicates the presence of fibrin.
By courtesy of the Department of Immunofluorescence, Institute of Dermatology, London, UK.
Differential diagnosis
Lichen planus should be differentiated from other diseases showing a lichenoid infiltrate and hydropic degeneration of the basal layer of the epithelium.130 Thus lichen planus may be indistinguishable from lichenoid keratosis and their distinction is entirely dependent on clinicopathological correlation. In many cases of lichenoid keratoses, there are other associated changes including focal spongiosis and parakeratosis. Atrophic lesions may be confused with poikiloderma and chronic discoid lupus erythematosus. A lichen planus-like morphology is typical of the early stages of chronic graft-versus-host disease (GVHD).
Lichen nitidus
Clinical features
Lichen nitidus is a rare but distinctive dermatosis, which shows an equal sex incidence.1 Children and young adults are predominantly affected. It presents clinically as an eruption of pinhead-sized, flesh-colored, shiny, flat-topped or dome-shaped papules and shows a predilection for the arms, chest, abdomen, and genitalia (Figs 7.37, 7.38).1–5 A positive Koebner’s phenomenon is typically present.5 The condition is usually localized and asymptomatic, although occasionally there may be mild or even intense pruritus.2 Rarely, generalized lesions have been described.2,6,7 An association with generalized lesions and Down’s syndrome has been documented, as has been a case after interferon-alpha therapy.8,9 Occasionally, papules may be encountered on the palms and soles.3–12 Familial cases have been rarely described.13,14 Lichen nitidus can spontaneously resolve within a few months or persist indefinitely.2
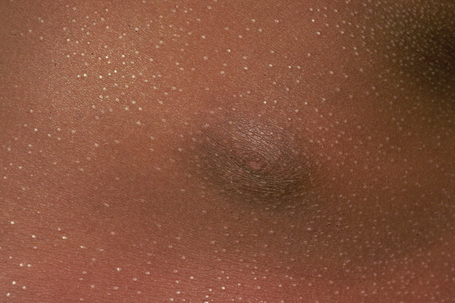
Fig. 7.37 Lichen nitidus: numerous tiny papules are present on the chest of a young child.
By courtesy of R.A. Marsden, MD, St George’s Hospital, London, UK.
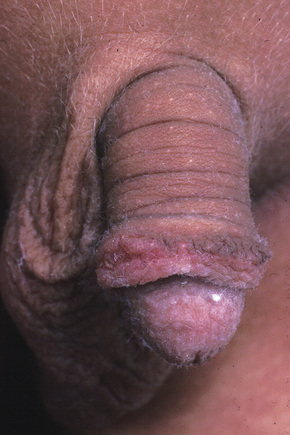
Fig. 7.38 Lichen nitidus: numerous tiny papules are present on the penis. The genitalia are commonly affected.
From the collection of the late N.P. Smith, MD, the Institute of Dermatology, London, UK.
Mucous membrane involvement presenting as grayish-yellow papules has also been described.4 Nail involvement, which is extremely rare, presents as thickening with ridges, rippling, terminal splitting, striations, and pits.2,4
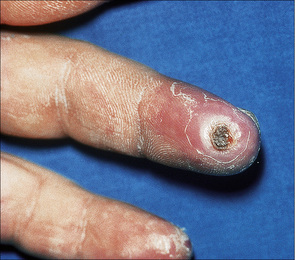
Keratodermic, vesicular, hemorrhagic, purpuric, and perforating variants may rarely be encountered.2,15–19 Perforating lichen nitidus shows a predilection for the forearms and fingers and may be trauma related.17,20
Actinic lichen nitidus refers to the development of lichen nitidus on sun-exposed sites, usually during the summer months. In some cases, involvement is predominantly facial and it may present in black patients.21 It shows considerable overlap with actinic lichen planus (see above).22,23
Histological features
Lichen nitidus is recognizable by a characteristic histology in many cases. The classic papule is sharply circumscribed and occupies the space of only four or five dermal papillae. It is often depressed in the center and composed of atrophic epidermis, frequently covered by a parakeratotic tier and overlying a cellular infiltrate (Figs 7.39–7.42). Clawlike extensions of epidermal ridges mark the lateral boundaries of the lesion. The epithelium shows basal cell hydropic degeneration, and cytoid bodies may be a feature. The inflammatory component consists of lymphocytes, histiocytes, and variable numbers of epithelioid cells. Giant cells are sometimes a feature and true granulomata may occasionally be found, although caseation is never present.24 In addition to red blood cell extravasation, purpuric variants may show increased vascularity with vessel wall thickening and hyalinization.18 In rare cases, a prominent lymphocytic inflammatory infiltrate can extend down the hair follicle and eccrine glands, making the distinction from lichen striatus challenging.24 A follicular variant of lichen nitidus may be seen and mimics lichen spinulosus histologically.25 However, rarely, lichen nitidus and lichen spinulosus may coexist clinically.26
Palmar lesions may be identical to those seen elsewhere or show a more diffuse bandlike upper dermal lymphohistiocytic infiltrate with associated giant cells and focal parakeratosis.3,10–12,27
Fibrin can be detected at the basement membrane zone by immunofluorescent techniques, but immunoglobulin deposition is not a feature.28,29 Immunophenotypic studies show that there is a marked excess of CD4+ cells (helper/inducer T cells) over CD8+ cells (cytotoxic/suppressor T cells).28 Langerhans cells are conspicuous.30 These findings are similar to those described for lichen planus.
Ultrastructural examination reveals rather non-specific findings including epidermal intercellular edema, subepidermal edema, colloid bodies, decreased numbers of desmosomes, and disruption or reduplication of the lamina densa.31–33 Perivascular electron-dense deposits (the nature of which is unknown) have been described in purpuric variants.8
Comment
Lichen nitidus may coexist with lichen planus or predate it and lichen nitidus-like lesions may be found in patients with typical lichen planus, but it is unlikely that the conditions are closely related.34,35 Wickham’s striae are not a feature of lichen nitidus and mucosal involvement is exceptional.2,4 Lichen nitidus is associated with parakeratosis and epidermal atrophy, in contrast to the orthohyperkeratosis and acanthosis seen in lichen planus. The saw-toothed appearance of the lower border of the epidermis seen in lichen planus is not a feature of lichen nitidus and immunofluorescence for immunogloblins is negative. Epithelioid cells and giant cells are characteristic of lichen nitidus and are not typically a feature of lichen planus. Three patients with Crohn’s disease were reported to develop lichen niditus; however it remains to be seen if lichen nitidus is truly an extragastrointestinal finding of this disease.36 Another patient developed lichen nitidus after hepatitis B vaccine injection.37 The significance of this is uncertain.
Lichenoid keratosis
Clinical features
Lichenoid keratosis (benign lichenoid keratosis, lichen planus-like keratosis, solitary lichen planus) is not uncommon and usually presents as a solitary, 0.3–2-cm diameter, sharply demarcated, erythematous, violaceous, tan or brown papule or plaque (Fig. 7.43).1,2 Occasionally, multiple lesions may be present.2,3 It is usually of short duration and shows a predilection for the face (particularly the cheeks and nose), neck, upper trunk (especially the presternal area), forearm, and dorsum of the hand.2,4–8 The surface is often scaly. Lesions are commonly asymptomatic, but mild pruritus has sometimes been documented.8 Patients are frequently Caucasian, but occasionally blacks are affected.2,7,8 Females develop these lesions more commonly than males, usually in their fourth to seventh decades.2,5
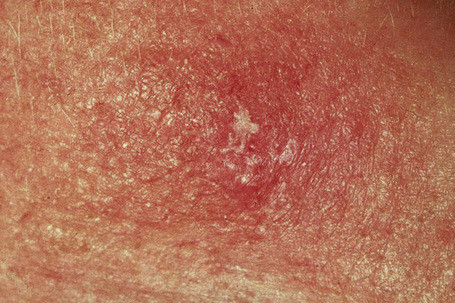
Fig. 7.43 Lichenoid keratosis: there is scaling overlying a slightly raised erythematous plaque.
By courtesy of the Institute of Dermatology, London.
Lichenoid keratosis is often clinically misdiagnosed as a seborrheic keratosis, superficial basal cell carcinoma, squamous cell carcinoma, actinic keratosis or Bowen’s disease.5
Pathogenesis and histological features
The precise nature of lichenoid keratosis is uncertain. In the past, it was regarded as a solitary lesion of lichen planus or thought to have an actinic pathogenesis.9–11 It was also proposed to represent an immunological or regressive response to a pre-existent epidermal lesion similar to the phenomenon encountered with a ‘halo’ nevus.2 The frequent association of solar lentigines or, less commonly, seborrheic keratoses in the adjacent epithelium has been cited as evidence in favor of this hypothesis.4–6,8 Recent studies have shown that the lymphocytic infiltrate in lichenoid keratosis to be immunophenotypically distinct from lichen planus. The lymphocytes in lichenoid keratosis are predominantly CD8-reactive in contrast to lichen planus. More CD20-positive B cells are usually seen in lichenoid keratosis. Furthermore, the lymphocyte infiltrate in lichenoid keratosis lacks the cutaneous lymphocyte antigen (CLA) expression, suggesting the absence of localized antigenic stimulation as seen in lichen planus.2,12 These studies suggest that lichenoid keratosis is an entity distinct from lichen planus despite the similarities in histology.
Despite this, histologically, as its name suggests, the features are similar to those of lichen planus. Thus there is hyperkeratosis, wedge-shaped hypergranulosis, variable acanthosis, and basal cell liquefactive degeneration sometimes accompanied by lymphocytic exocytosis (Figs. 7.44, 7.45).2,3,5 Foci of parakeratosis are also frequently seen.1,2 Although the saw-toothed acanthosis of lichen planus is sometimes evident, more often the epithelium merely shows broadened, widened, and irregular epidermal ridges.4 The basal epidermal layers may sometimes show very minor degrees of cytological atypia, including cellular and nuclear enlargement with conspicuous nucleoli, but these changes represent regenerative phenomena.3 Dysplasia as seen in lichenoid actinic keratosis is not a feature of a lichenoid keratosis. Colloid bodies are usually conspicuous in both the epidermis and dermis and pigmentary incontinence is often marked (Figs 7.46–7.48).1,2,7 Apoptotic keratinocytes can be prominent and may be associated with inatraepidermal blister formation with subepidermal vesiculation. Epidermal pallor and dermal edema can be seen in cases with only slight or no acanthosis and an interface population of lymphocytes along the junction with vacuolar degeneration. Foci of atrophy can be occasionally encountered.2 In some cases a combination of lichenoid and spongiotic changes may be seen.
A dense chronic inflammatory cell infiltrate is typically present in the superficial dermis. Although this characteristically has a lichenoid distribution, on some occasions it may be more discrete and predominantly perivascular in location.4,7 The infiltrate consists largely of lymphocytes and histiocytes, but small numbers of plasma cells and eosinophils are occasionally present. Exceptionally, a few atypical lymphocytes (enlarged with hyperchromatic, irregular, contoured nuclei) which are CD30 and CD3 reactive can be also seen.2 The adjacent dermis sometimes shows lentigo and solar elastosis, which is usually mild if present. Features suggestive of mycoses fungoides such as Pautrier abscesses, dermal–epidermal tagging and mild lymphocytic atypia have been rarely noted in benign lichenoid keratosis.13 Clinicopathological correlation and careful follow-up are essential in such cases to avoid misdiagnosis.
Immunofluorescence findings, which are similar to those of lichen planus, comprise deposits of IgM and, less commonly, IgG outlining cytoid bodies.5
Differential diagnosis
If clinical information is available, differentiation from lichen planus should present little difficulty. Lichen planus is characterized by large numbers of lesions in contradistinction to the single papule or plaque of lichenoid keratosis. In addition, lichen planus is usually itchy. Parakeratosis and dermal plasma cells with eosinophils are not a feature of lichen planus, but are typical of lichenoid keratosis.7
Both actinic keratoses and squamous cell carcinoma in situ may sometimes show a lichenoid inflammatory cell reaction. Dysplasia by definition is not a feature of lichenoid keratosis.1,2 Inflamed seborrheic keratosis and porokeratosis can have a prominent lichenoid reaction. The absence of horn cyst formation, squamous epidermal eddies, and laminated stratum corneum keratin helps distinguish these lesions from seborrheic keratosis, while the absence of cornoid lamella excludes porokeratosis. Melanocytic lesions with halo phenomenon can become a diagnostic consideration and require examination of the dermis and dermoepidermal junction for melanocytic nests. In difficult cases, additional step sections or S-100 protein immunohistochemical study can prove useful. Finally, the presence of scattered CD30-positive lymphocytes in some cases of lichenoid keratosis may raise the histological differential diagnosis of lymphomatoid papulosis. However, the paucity of these enlarged CD30-positive cells, the absence of a deep infiltrate, and the clinically history of a solitary lesion is reassuring for lichenoid keratosis.2
Lichen striatus
Clinical features
Lichen striatus (Blaschko linear acquired inflammatory skin eruption (BLAISE)) is an uncommon, usually asymptomatic, dermatosis of unknown etiology, affecting the limbs or neck in which lesions typically follow Blaschko’s lines.1–8 Infrequently, the condition is pruritic.6–9 It is self-limiting, normally disappearing within months to a year of onset. It shows a female predominance (2–3:1) and, although it may occur at any age, it most often presents in children aged 5–15 years.2,5,7,8 Rarely, lichen striatus has been described in adults (adult Blaschkitis, see below).4,10,11 Occurrence during pregnancy is very rare.12 A family history is rarely encountered, suggesting a genetic predisposition and/or a common environmental etiology in such cases.2,6,8,13,14 It is associated with seasonal variation with most series reporting the majority of patients presenting in spring and summer,2,7 with the exception of one large series where the majority of patients presented in the winter 8,13 Case clustering has been documented.2
Lesions, usually solitary and unilateral, present as erythematous or flesh-colored lichenoid or sometimes psoriasiform scaly papules, which coalesce into a continuous or interrupted linear or curved band, 1–3 cm wide and often covering the whole length of a limb, either lower or upper extremities (Figs 7.49, 7.50).2,8 Occasionally, multiple lesions have been recorded, as has bilaterality.8,15,16 Presentation at two different sites and at multiple sites may exceptionally occur.17 Nail changes, which may affect a single nail, include onycholysis, longitudinal ridging, splitting, and nail loss.8,1,18,19 An exceptional case of lichen striatus with bilateral nail dystrophy has been described.20 Lichen striatus is not associated with Koebner’s phenomenon. Hypo- or hyperpigmentation sometimes follows resolution, which may be marked in people with pigmented skin.8 Lichen striatus is associated with atopy in up to 60% of patients.1,6–8
< div class='tao-gold-member'>
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree