Objectives
- Defines diffusion, and distinguishes it from “bulk flow.”
- States Fick’s law for diffusion.
- Distinguishes between perfusion limitation and diffusion limitation of gas transfer in the lung.
- Describes the diffusion of oxygen from the alveoli into the blood.
- Describes the diffusion of carbon dioxide from the blood to the alveoli.
- Defines the diffusing capacity and discusses its measurement.
- Interprets standard pulmonary function test (PFT) data.
Diffusion of Gases and Interpretation of Pulmonary Function Tests: Introduction
Diffusion of a gas occurs when there is a net movement of molecules from an area in which that particular gas exerts a high partial pressure to an area in which it exerts a lower partial pressure. Movement of a gas by diffusion is therefore different from the movement of gases through the conducting airways, which occurs by “bulk flow” (mass movement or convection). During bulk flow, gas movement results from differences in total pressure, and molecules of different gases move together along the total pressure gradient. During diffusion, different gases move according to their own individual partial pressure gradients. Gas transfer during diffusion occurs by random molecular movement. It is therefore dependent on temperature because molecular movement increases at higher temperatures. Gases move in both directions during diffusion, but the area of higher partial pressure, because of its greater number of molecules per unit volume, has proportionately more random “departures.” Thus, the net movement of gas is dependent on the partial pressure difference between the 2 areas. In a static situation, diffusion continues until no partial pressure differences exist for any gases in the 2 areas; in the lungs, oxygen and carbon dioxide continuously enter and leave the alveoli, and so such an equilibrium does not take place.
Fick’s Law for Diffusion
Oxygen is brought into the alveoli by bulk flow through the conducting airways. When air flows through the conducting airways during inspiration, the linear velocity of the bulk flow decreases as the air approaches the alveoli. This is because the total cross-sectional area increases dramatically in the distal portions of the tracheobronchial tree, as was seen in Figure 1–2. The linear velocity of bulk flow through a tube is equal to the flow divided by the cross sectional area:
By the time the air reaches the alveoli, bulk flow probably ceases, and further gas movement occurs by diffusion. Oxygen then moves through the gas phase in the alveoli according to its own partial pressure gradient. The distance from the alveolar duct to the alveolar-capillary interface is usually less than 1 mm. Diffusion in the alveolar gas phase is believed to be greatly assisted by the pulsations of the heart and blood flow, which are transmitted to the alveoli and increase molecular motion.
Oxygen then diffuses through the alveolar-capillary interface. It must first, therefore, move from the gas phase to the liquid phase, according to Henry’s law, which states that the amount of a gas absorbed by a liquid with which it does not combine chemically is directly proportional to the partial pressure of the gas to which the liquid is exposed and the solubility of the gas in the liquid. Oxygen must dissolve in and diffuse through the thin layer of pulmonary surfactant, the alveolar epithelium, the interstitium, and the capillary endothelium, as was shown in Figure 1–5 (step 2, near the arrow). It must then diffuse through the plasma (step 3), where some remains dissolved and the majority enters the erythrocyte and combines with hemoglobin (step 4). The blood then carries the oxygen out of the lung by bulk flow and distributes it to the other tissues of the body, as shown in Figure 1–1. At the tissues, oxygen diffuses from the erythrocyte through the plasma, capillary endothelium, interstitium, tissue cell membrane, and cell interior and into the mitochondrial membrane. The process is almost entirely reversed for carbon dioxide, as shown in Figure 1–1.
The factors that determine the rate of diffusion of gas through the alveolar-capillary barrier are described by Fick’s law for diffusion, shown here in a simplified form:
- A = surface area of the barrier available for diffusion
- D = diffusion coefficient, or diffusivity, of the particular gas in the barrier
- T = thickness of barrier of the diffusion distance
- P1 − P2 = partial pressure difference of the gas across the barrier
![]() That is, the volume of gas per unit of time moving across the alveolar-capillary barrier is directly proportional to the surface area of the barrier, the diffusivity, and the difference in concentration between the 2 sides, but is inversely proportional to the barrier thickness.
That is, the volume of gas per unit of time moving across the alveolar-capillary barrier is directly proportional to the surface area of the barrier, the diffusivity, and the difference in concentration between the 2 sides, but is inversely proportional to the barrier thickness.
The surface area of the blood-gas barrier is believed to be at least 70 m2 in a healthy average-sized adult at rest. That is, about 70 m2 of the potential surface area is both ventilated and perfused at rest. If more capillaries are recruited, as in exercise, the surface area available for diffusion increases; if venous return falls, for example, because of hemorrhage, or if alveolar pressure is raised by positive-pressure ventilation, then capillaries may be derecruited and the surface area available for diffusion may decrease.
The thickness of the alveolar-capillary diffusion barrier is only about 0.2 to 0.5 μm. This barrier thickness can increase in interstitial fibrosis or interstitial edema, thus interfering with diffusion. Diffusion probably increases at higher lung volumes because as alveoli are stretched, the diffusion distance decreases slightly (and also because small airways subject to closure may be open at higher lung volumes).
The diffusivity, or diffusion constant, for a gas is directly proportional to the solubility of the gas in the diffusion barrier and is inversely proportional to the square root of the molecular weight (MW) of the gas:
The relationship between solubility and diffusion through the barrier has already been discussed. The diffusivity is inversely proportional to the square root of the MW of the gas because different gases with equal numbers of molecules in equal volumes have the same molecular energy if they are at the same temperature. Therefore, light molecules travel faster, have more frequent collisions, and diffuse more rapidly. Thus, Graham’s law states that the relative rates of diffusion of 2 gases are inversely proportional to the square roots of their MWs, if all else is equal.
Because the relative diffusion rates in the gas phase are inversely proportional to the ratio of the square roots of their MWs,
That is, because oxygen is less dense than carbon dioxide, it should diffuse 1.2 times as fast as carbon dioxide (which it does as it moves through the alveoli). In the alveolar-capillary barrier, however, the relative solubilities of oxygen and carbon dioxide must also be considered. The solubility of carbon dioxide in the liquid phase is about 24 times that of oxygen, and so carbon dioxide diffuses about 0.85 × 24, or about 20 times, more rapidly through the alveolar-capillary barrier than does oxygen. For this reason, patients develop problems in oxygen diffusion through the alveolar-capillary barrier before carbon dioxide retention due to diffusion impairment occurs.
Limitations of Gas Transfer
The factors that limit the movement of a gas through the alveolar-capillary barrier, as described by Fick’s law for diffusion, can be divided into 3 components: the diffusion coefficient, the surface area and thickness of the alveolar-capillary membrane, and the partial pressure difference across the barrier for each particular gas. The diffusion coefficient, as discussed in the previous section, is dependent on the physical properties of the gases and the alveolar-capillary membrane. The surface area and thickness of the membrane are physical properties of the barrier, but they can be altered by changes in the pulmonary capillary blood volume, the cardiac output, or the pulmonary artery pressure; by changes in lung volume; or by diseases such as fibrosis or emphysema. The partial pressure difference of a gas (across the barrier) is the final major determinant of its rate of diffusion. The partial pressure of a gas in the mixed venous blood and in the pulmonary capillaries is just as important a factor as its alveolar partial pressure in determining its rate of diffusion. This will be demonstrated in the next section.
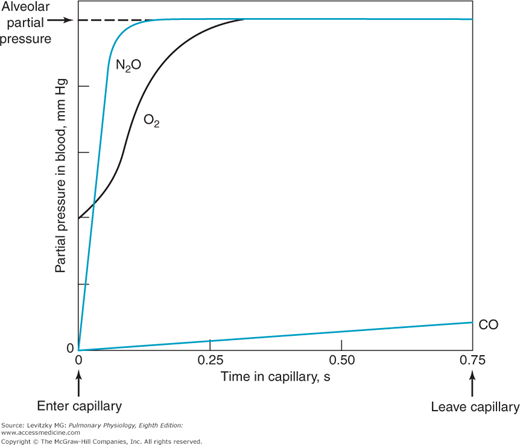
An erythrocyte and its attendant plasma spend an average of about 0.75 to 1.2 seconds inside the pulmonary capillaries at resting cardiac outputs. This time can be estimated by dividing the pulmonary capillary blood volume by the pulmonary blood flow (expressed in milliliters per second). Some erythrocytes may take less time to traverse the pulmonary capillaries; others may take longer. Figure 6–1 shows schematically the calculated change with time in the partial pressures in the blood of 3 gases: oxygen, carbon monoxide, and nitrous oxide. These are shown in comparison to the alveolar partial pressures for each gas, as indicated by the dotted line. This alveolar partial pressure is different for each of the 3 gases, and it depends on its concentration in the inspired gas mixture and on how rapidly it is removed by the pulmonary capillary blood. The schematic is drawn as though all 3 gases were administered simultaneously, but this is not necessarily the case. Consider each gas as though it were acting independently of the others.
Figure 6–1.
Calculated changes in the partial pressures of carbon monoxide, nitrous oxide, and oxygen in the blood as it passes through a functional pulmonary capillary. There are no units on the ordinate because the scale is different for each of the 3 gases, depending on the alveolar partial pressure of each gas. The abscissa is in seconds, indicating the time the blood has spent in the capillary. At resting cardiac outputs, blood spends an average of 0.75 of a second in a pulmonary capillary. The alveolar partial pressure of each gas is indicated by the dotted line. Note that the partial pressures of nitrous oxide and oxygen equilibrate rapidly with their alveolar partial pressure. (Reproduced with permission from Comroe, 1962.)
The partial pressure of carbon monoxide in the pulmonary capillary blood rises very slowly compared with that of the other 2 gases in the figure. (Obviously, a low inspired concentration of carbon monoxide must be used for a very short time in such an experiment.) Nevertheless, if the content of carbon monoxide (in milliliters of carbon monoxide per milliliter of blood) were measured simultaneously, it would be rising very rapidly. The reason for this rapid rise is that carbon monoxide combines chemically with the hemoglobin in the erythrocytes. Indeed, the affinity of carbon monoxide for hemoglobin is about 210 times that of oxygen for hemoglobin. The carbon monoxide that is chemically combined with hemoglobin does not contribute to the partial pressure of carbon monoxide in the blood because it is no longer physically dissolved in it. ![]() Therefore, the partial pressure of carbon monoxide in the pulmonary capillary blood does not come close to the partial pressure of carbon monoxide in the alveoli during the time that the blood is exposed to the alveolar carbon monoxide. (If the alveolar partial pressure of carbon monoxide were great enough to saturate the hemoglobin, the pulmonary capillary partial pressure would rise rapidly.) The partial pressure difference across the alveolar-capillary barrier for carbon monoxide is thus well maintained for the entire time the blood spends in the pulmonary capillary, and the diffusion of carbon monoxide is limited only by its diffusivity in the barrier and by the surface area and thickness of the barrier—that is, the diffusion characteristics of the barrier itself. Carbon monoxide transfer from the alveolus to the pulmonary capillary blood is referred to as diffusion-limited rather than perfusion-limited.
Therefore, the partial pressure of carbon monoxide in the pulmonary capillary blood does not come close to the partial pressure of carbon monoxide in the alveoli during the time that the blood is exposed to the alveolar carbon monoxide. (If the alveolar partial pressure of carbon monoxide were great enough to saturate the hemoglobin, the pulmonary capillary partial pressure would rise rapidly.) The partial pressure difference across the alveolar-capillary barrier for carbon monoxide is thus well maintained for the entire time the blood spends in the pulmonary capillary, and the diffusion of carbon monoxide is limited only by its diffusivity in the barrier and by the surface area and thickness of the barrier—that is, the diffusion characteristics of the barrier itself. Carbon monoxide transfer from the alveolus to the pulmonary capillary blood is referred to as diffusion-limited rather than perfusion-limited.