64
Dietary Supplements & Herbal Medications*
CASE STUDY
A 65-year-old man with a history of coronary artery disease, high cholesterol, type 2 diabetes, and hypertension presents with a question about a dietary supplement. He is in good health, exercises regularly, and eats a low-fat, low-salt diet. His most recent laboratory values show that his low-density lipoprotein (LDL) cholesterol is still slightly above goal at 120 mg/dL (goal < 100 mg/dL) and his hemoglobin A1c is well controlled at 6%. His blood pressure is also well controlled. His medications include simvastatin, metformin, benazepril, and aspirin. He also regularly takes a vitamin B-complex supplement and coenzyme Q10. He asks you if taking a garlic supplement could help to bring his LDL cholesterol down to less than 100 mg/dL. What are two rationales for why he might be using a coenzyme Q10 supplement? Are there any supplements that could increase bleeding risk if taken with aspirin?
The medical use of plants in their natural and unprocessed form undoubtedly began when the first intelligent animals noticed that certain food plants altered particular body functions. While there is a great deal of historical information about the use of plant-based supplements, there is also much unreliable information from poorly designed clinical studies that do not account for randomization errors, confounders, and—most importantly—a placebo effect that can contribute 30–50% of the observed response. Since the literature surrounding dietary supplements is evolving, reputable evidence-based resources should be used to evaluate claims and guide treatment decisions. An unbiased and regularly updated compendium of basic and clinical reports regarding botanicals is Pharmacist’s Letter/Prescriber’s Letter Natural Medicines Comprehensive Database (see References). Another evidence-based resource is Natural Standard, which includes an international, multi-disciplinary collaborative website, http://www.naturalstandard.com. The recommendations in this database are limited by the quality of the existing research available for each dietary supplement ingredient. (These two sources may be combined in the near future.) As a result, all statements regarding positive benefits should be regarded as preliminary and conclusions regarding safety should be considered tentative at this time.
For legal purposes, “dietary supplements” are distinguished from “prescription drugs” derived from plants (morphine, digitalis, atropine, etc) by virtue of being available without a prescription and, unlike “over-the-counter medications,” are legally considered dietary supplements rather than drugs. This distinction eliminates the need for proof of efficacy and safety prior to marketing and also places the burden of proof on the FDA to prove that a supplement is harmful before its use can be restricted or removed from the market. Furthermore, marketed dietary supplements are not tested for dose-response relationships or toxicity and there is a lack of adequate testing for mutagenicity, carcinogenicity, and teratogenicity. Although manufacturers are prohibited from marketing unsafe or ineffective products, the FDA has met significant challenges from the supplement industry largely due to the strong lobbying effort by supplement manufacturers and the variability in interpretation of the Dietary Supplement Health and Education Act (DSHEA). The DSHEA defines dietary supplements as vitamins, minerals, herbs or other botanicals, amino acids or dietary supplements used to supplement the diet by increasing dietary intake, or concentrates, metabolites, constituents, extracts, or any combination of these ingredients. For the purposes of this chapter, plant-based substances and certain synthetic purified chemicals will be referred to as dietary supplements. Among the purified chemicals, glucosamine, coenzyme Q10, and melatonin are of significant pharmacologic interest.
This chapter provides some historical perspective and describes the evidence provided by randomized, double-blind, placebo-controlled trials, meta-analyses, and systematic reviews involving several of the most commonly used agents in this class. Ephedrine, the active principle in Ma-huang, is discussed in Chapter 9.
HISTORICAL & REGULATORY FACTORS
Under the DSHEA, dietary supplements are not considered over-the-counter drugs in the USA but rather food supplements used for health maintenance. Legally, dietary supplements are intended to supplement the diet, but consumers may use them in the same fashion as drugs and even use them in place of drugs or in combination with drugs.
In 1994, the U.S. Congress, influenced by growing “consumerism” as well as strong manufacturer lobbying efforts, passed the DSHEA. The DSHEA required the establishment of Good Manufacturing Practice (GMP) standards for the supplement industry; however, it was not until 2007 that the FDA issued a final rule on the proposed GMP standards. This 13-year delay allowed supplement manufacturers to self-regulate the manufacturing process and resulted in many instances of adulteration, misbranding, and contamination. For example, a recent study using DNA barcoding to confirm botanical content evaluated 44 botanicals containing 30 plant species and found product substitutions in 32% of samples (see Newmaster reference). Therefore, much of the criticism regarding the dietary supplement industry involves problems with botanical misidentification, a lack of product purity, and variations in potency and purification, which continue to be problematic even with GMP standards in place. When the new GMP standards are met, dietary supplement manufacturers should be in compliance with this legislation. However, the FDA has limited resources to investigate and oversee compliance with manufacturing standards, particularly since many ingredient suppliers are based overseas. Furthermore, the dietary supplement ingredient supply chain is complex and federal regulators are not able to inspect all manufacturing facilities in a timely and efficient manner.
Because of the problems that resulted from self-regulation, another law, the Dietary Supplement and Non-Prescription Drug Consumer Protection Act, was approved in 2006. This law requires manufacturers, packers, or distributors of supplements to submit reports of serious adverse events to the FDA. Serious adverse events are defined as death, a life-threatening event, hospitalization, a persistent or significant disability or incapacity, congenital anomaly or birth defect, or an adverse event that requires medical or surgical intervention to prevent such outcomes based on reasonable medical judgment. These reports are intended to identify trends in adverse effects and would help to alert the public to safety issues.
CLINICAL ASPECTS OF THE USE OF BOTANICALS
Many U.S. consumers have embraced the use of dietary supplements as a “natural” approach to their health care. Unfortunately, misconceptions regarding safety and efficacy of the agents are common, and the fact that a substance can be called “natural” does not of course guarantee its safety. In fact, botanicals may be inherently inert or toxic. If a manufacturer does not follow GMP this can also result in intentional or unintentional plant species substitutions (eg, misidentification), adulteration with pharmaceuticals, or contamination.
Adverse effects have been documented for a variety of dietary supplements; however, under-reporting of adverse effects is likely since consumers do not routinely report, and do not know how to report an adverse effect if they suspect that the event was caused by consumption of a supplement. Furthermore, chemical analysis is rarely performed on the products involved, including those products that are described in the literature as being linked to an adverse event. This leads to confusion about whether the primary ingredient or an adulterant caused the adverse effect. In some cases, the chemical constituents of the herb can clearly lead to toxicity. Some of the herbs that should be used cautiously or not at all are listed in Table 64–1.
TABLE 64–1 Various supplements and some associated risks.
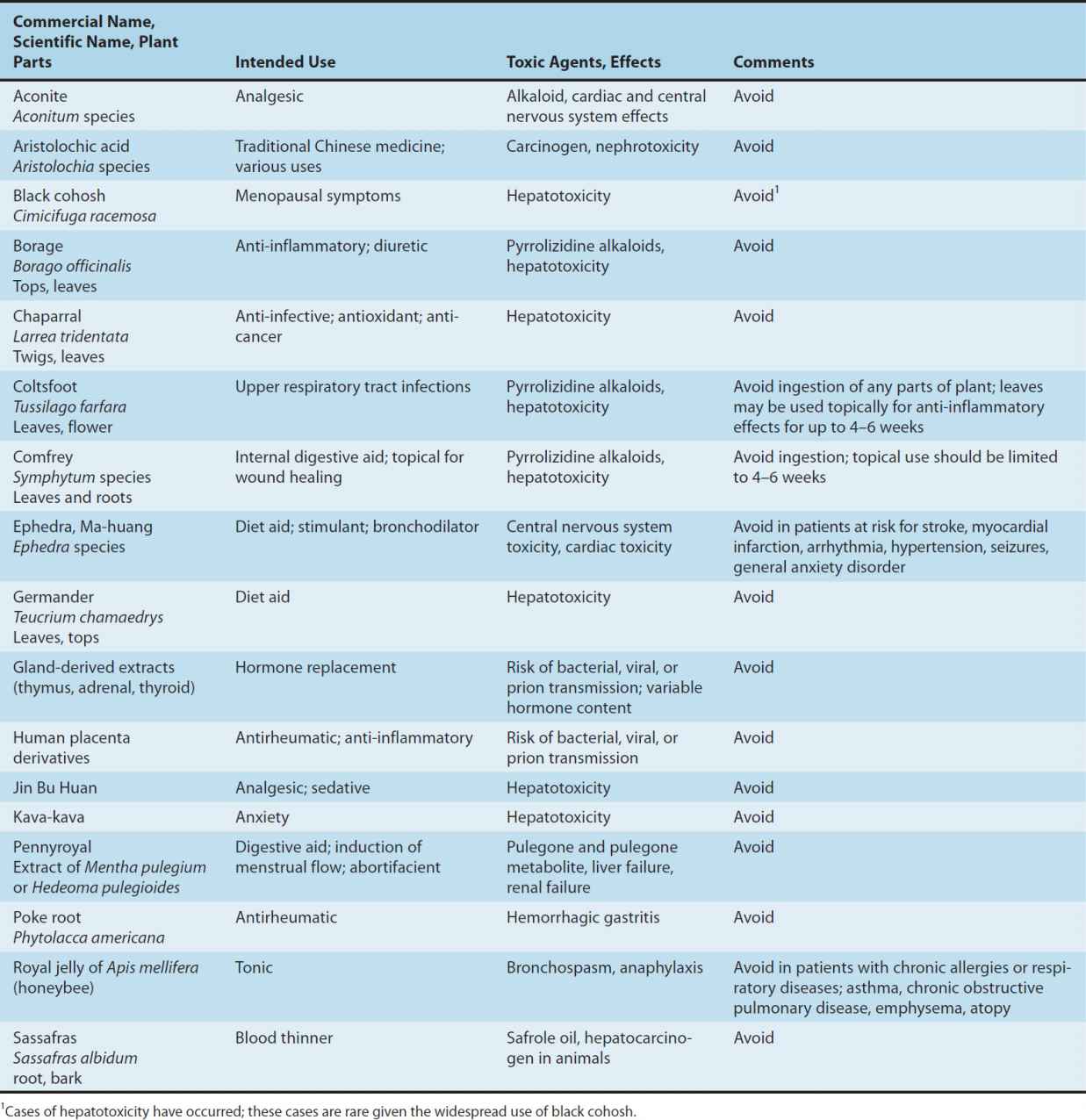
An important risk factor in the use of dietary supplements is the lack of adequate testing for drug interactions. Since botanicals may contain hundreds of active and inactive ingredients, it is very difficult and costly to study potential drug interactions when they are combined with other medications. This may present significant risks to patients.
 BOTANICAL SUBSTANCES
BOTANICAL SUBSTANCES
ECHINACEA (ECHINACEA PURPUREA)
Chemistry
The three most widely used species of Echinacea are Echinacea purpurea, E pallida, and E angustifolia. The chemical constituents include flavonoids, lipophilic constituents (eg, alkamides, polyacetylenes), water-soluble polysaccharides, and water-soluble caffeoyl conjugates (eg, echinacoside, cichoric acid, caffeic acid). Within any marketed echinacea formulation, the relative amounts of these components are dependent upon the species used, the method of manufacture, and the plant parts used. E purpurea, the purple coneflower, has been the most widely studied in clinical trials. Although the active constituents of echinacea are not completely known, cichoric acid from E purpurea and echinacoside from E pallida and E angustifolia, as well as alkamides and polysaccharides, are most often noted as having immune-modulating properties. Most commercial formulations, however, are not standardized for any particular constituent.
Pharmacologic Effects
1. Immune modulation—The effect of echinacea on the immune system is controversial. In vivo human studies using commercially marketed formulations of E purpurea have shown increased phagocytosis, total circulating monocytes, neutrophils, and natural killer cells, indicative of general immune modulation. In vitro, a standardized ethanol extract of the aerial (above-ground) parts of E purpurea, known as Echinaforce, inhibited the rise in pro-inflammatory cytokines and interleukins-6 and -8, and also inhibited mucin secretion caused by exposure to rhinovirus type 1A in a 3D tissue model of human airway epithelium. This type of model is intended to mimic what would be seen in vivo. The extract had no effect on cytokine actions.
2. Anti-inflammatory effects—Certain echinacea constituents have demonstrated anti-inflammatory properties in vitro. Inhibition of cyclooxygenase, 5-lipoxygenase, and hyaluronidase may be involved. In animals, application of E purpurea prior to application of a topical irritant reduced both paw and ear edema. Despite these laboratory findings, randomized, controlled clinical trials involving echinacea for wound healing have not been performed in humans.
3. Antibacterial, antifungal, antiviral, and antioxidant effects—In vitro studies have reported some antibacterial, antifungal, antiviral, and antioxidant activity with echinacea constituents. For example, Echinaforce demonstrated virucidal activity (MIC100 < 1 μg/mL) against influenza and herpes simplex viruses and bactericidal activity against Streptococcus pyogenes, Haemophilus influenzae, and Legionella pneumophila in human bronchial cells. In vitro, Echinaforce inactivated both avian influenza virus (H5N1, H7N7) and swine-origin influenza virus (H1N1) at doses consistent with recommended oral consumption. The extract blocked key steps (ie, viral hemagglutination activity and neuraminidase activity in vitro) involved in early virus replication and cellular entry. It was less effective against intracellular virus. Newer in vitro research in human skin fibroblasts also suggests bactericidal activity and inhibition of secretion of inflammatory cytokines produced by Propionibacterium acnes with Echinaforce.
Clinical Trials
Echinacea is most often used to enhance immune function in individuals who have colds and other respiratory tract infections. Two reviews have assessed the efficacy of echinacea for this primary indication. A review by the Cochrane Collaboration involved 24 randomized, double-blind trials with 33 comparisons of Echinacea mono-preparations and placebo. Trials were included if they involved echinacea for cold treatment or prevention, where the primary efficacy outcome was cold incidence in prevention trials and duration of symptoms in treatment trials. Overall, the review did not find significant evidence of benefit for Echinacea (among all species) in treating colds. Preparations made from the aerial parts of E purpurea plants and prepared as alcoholic extracts or pressed juices were discussed as possibly being preferred to other formulations for cold treatment in adults, but still having a weak overall treatment effect. In prevention trials, pooling results suggested a small relative risk reduction in development of 10–20%, but no statistically significant benefit within individual trials.
A separate meta-analysis involving 14 randomized, placebo-controlled trials of echinacea for cold treatment or prevention was published in Lancet. In this review, echinacea decreased the risk of developing clear signs and symptoms of a cold by 58% and decreased symptom duration by 1.25 days. This review, however, was confounded by the inclusion of four clinical trials involving multi-ingredient echinacea preparations, as well as three studies using rhinovirus inoculation versus natural cold development.
Echinacea has been used investigationally to enhance hematologic recovery following chemotherapy. It has also been used as an adjunct in the treatment of urinary tract and vaginal fungal infections. These indications require further research before they can be accepted in clinical practice. E purpurea is ineffective in treating recurrent genital herpes.
Adverse Effects
Adverse effects with oral commercial formulations are minimal and most often include unpleasant taste, gastrointestinal upset, or rash. In one large clinical trial, pediatric patients using an oral echinacea product were significantly more likely to develop a rash than those taking placebo.
Drug Interactions & Precautions
Until the role of echinacea in immune modulation is better defined, this agent should be avoided in patients with immune deficiency disorders (eg, AIDS, cancer), or autoimmune disorders (eg, multiple sclerosis, rheumatoid arthritis). While there are no reported drug interactions for echinacea, in theory, it should also be avoided in persons taking immunosuppressant medications (eg, organ transplant recipients).
Dosage
It is recommended to follow the dosing on the package label, as there may be variations in dose based on the procedure used in product manufacture. Standardized preparations made from the aerial parts of E purpurea (Echinaforce, Echinaguard) as an alcoholic extract or fresh pressed juice may be preferred in adults for common cold treatment if taken within the first 24 hours of cold symptoms. It should not be used on a continuous basis for longer than 10–14 days.
GARLIC (ALLIUM SATIVUM)
Chemistry
The pharmacologic activity of garlic involves a variety of organosulfur compounds. Dried and powdered formulations contain many of the compounds found in raw garlic and will usually be standardized to allicin or alliin content. Allicin is responsible for the characteristic odor of garlic, and alliin is its chemical precursor. Dried powdered formulations are often enteric-coated to protect the enzyme allinase (the enzyme that converts alliin to allicin) from degradation by stomach acid. Aged garlic extract has also been studied in clinical trials, but to a lesser degree than dried, powdered garlic. Aged garlic extract contains no alliin or allicin and is odor-free. Its primary constituents are water-soluble organosulfur compounds, and packages may carry standardization to the compound S-allylcysteine.
Pharmacologic Effects
1. Cardiovascular effects—In vitro, allicin and related compounds inhibit HMG-CoA reductase, which is involved in cholesterol biosynthesis (see Chapter 35), and exhibit antioxidant properties. Several clinical trials have investigated the lipid-lowering potential of garlic. A meta-analysis by Reinhart and colleagues involved 29 randomized, double-blind, placebo-controlled trials and found a small but significant reduction in both total cholesterol (−0.19 mmol/L, 6 mg/dL) and triglycerides (−0.011 mmol/L, 1.1 mg/dL), but no effect on low- (LDL) or high-density lipoproteins (HDL). A more recent meta-analysis of 26 randomized, double-blind, placebo-controlled trials found a significant reduction in total cholesterol (−0.28 mmol/L, 9.3 mg/dL) for garlic compared with placebo. No impact on LDL or HDL was observed. Trials of longer duration (> 12 weeks) showed a greater reduction in total cholesterol and triglycerides as compared to trials of shorter duration (0–4 weeks), with garlic powder and aged garlic extract formulations having the greatest benefit. Cumulatively, these data suggest a small but significant benefit of garlic in lowering total cholesterol and triglycerides. The lack of change in HDL and LDL indicate that garlic is unlikely to be clinically relevant, however, in benefiting patients with hyperlipidemia.
Clinical trials report antiplatelet effects (possibly through inhibition of thromboxane synthesis or stimulation of nitric oxide synthesis) following garlic ingestion. A majority of human studies also suggest enhancement of fibrinolytic activity. These effects in combination with antioxidant effects (eg, increased resistance to LDL oxidation) and reductions in total cholesterol might be beneficial in patients with atherosclerosis. A randomized, controlled trial among persons with advanced coronary artery disease who consumed dried powdered garlic for 4 years showed significant reductions in secondary markers (plaque accumulation in the carotid and femoral arteries) as compared with placebo, but primary end points (death, stroke, myocardial infarction) were not assessed.
Garlic constituents may affect blood vessel elasticity and blood pressure. Various mechanisms have been proposed. There have been a limited number of randomized, controlled trials in humans for this indication. Ten trials were included in a systematic review and meta-analysis that found no effect on systolic or diastolic pressure in patients without elevated systolic blood pressure but a significant reduction in systolic and diastolic pressure among the three trials involving patients with elevated systolic blood pressure. A Cochrane review on the effect of garlic monotherapy for prevention of cardiovascular morbidity and mortality in hypertensive patients identified a small number of randomized, controlled trials for inclusion. Although the trials lacked outcomes to assess an impact on cardiovascular events, the review did identify a significant reduction in systolic and diastolic pressure compared with placebo. A separate Cochrane review of the effect of garlic on peripheral occlusive disease found insufficient support for this indication.
2. Endocrine effects—The effect of garlic on glucose homeostasis does not appear to be significant in persons with diabetes. Certain organosulfur constituents in garlic, however, have demonstrated hypoglycemic effects in nondiabetic animal models.
3. Antimicrobial effects—The antimicrobial effect of garlic has not been extensively studied in clinical trials. Allicin has been reported to have in vitro activity against some gram-positive and gram-negative bacteria as well as fungi (Candida albicans), protozoa (Entamoeba histolytica), and certain viruses. The primary mechanism involves the inhibition of thiol-containing enzymes needed by these microbes. Given the availability of safe and effective prescription antimicrobials, the usefulness of garlic in this area appears limited.
4. Antineoplastic effects—In rodent studies, garlic inhibits procarcinogens for colon, esophageal, lung, breast, and stomach cancer, possibly by detoxification of carcinogens and reduced carcinogen activation. Several epidemiologic case-control studies demonstrate a reduced incidence of stomach, esophageal, and colorectal cancers in persons with high dietary garlic consumption. Current anti-cancer studies are focused on specific organosulfur garlic compounds in in vivo animal models of cancer, and in vitro effects on human cancer cell lines.
Adverse Effects
Following oral ingestion, adverse effects of garlic products may include nausea (6%), hypotension (1.3%), allergy (1.1%), and bleeding (rare). Breath and body odor have been reported with an incidence of 20–40% at recommended doses using enteric-coated powdered garlic formulations. Contact dermatitis may occur with the handling of raw garlic.
Drug Interactions & Precautions
Because of reported antiplatelet effects, patients using anticlotting medications (eg, warfarin, aspirin, ibuprofen) should use garlic cautiously. Additional monitoring of blood pressure and signs and symptoms of bleeding is warranted. Garlic may reduce the bioavailability of saquinavir, an antiviral protease inhibitor, but it does not appear to affect the bioavailability of ritonavir.
Dosage
Dried, powdered garlic products should be standardized to contain 1.3% alliin (the allicin precursor) or have an allicin-generating potential of 0.6%. Enteric-coated formulations are recommended to minimize degradation of the active substances. A daily dose of 600–900 mg/d of powdered garlic is most common. This is equivalent to one clove of raw garlic (2–4 g) per day. A garlic bulb can contain up to 1.8% alliin.
GINKGO (GINKGO BILOBA)
Chemistry
Ginkgo biloba extract is prepared from the leaves of the ginkgo tree. The most common formulation is prepared by concentrating 50 parts of the crude leaf to prepare one part of extract. The active constituents in ginkgo are flavone glycosides and terpenoids including ginkgolides A, B, C, J, and bilobalide.
Pharmacologic Effects
1. Cardiovascular effects—In animal models and some human studies, ginkgo has been shown to increase blood flow, reduce blood viscosity, and promote vasodilation, thus enhancing tissue perfusion. Enhancement of endogenous nitric oxide effects (see Chapter 19) and antagonism of platelet-activating factor have been observed in animal models.
Ginkgo biloba has been studied for its effects on mild to moderate occlusive peripheral arterial disease. Among 11 randomized, placebo-controlled studies involving 477 participants using standardized ginkgo leaf extract (EGb761) for up to 6 months, a nonsignificant trend toward improvements in pain-free walking distance (increase of 64.5 meters) was observed (p = .06). The authors concluded that the standardized extract lacked benefit for this indication.
The Ginkgo Evaluation of Memory (GEM) study and the recently published GuidAge study evaluated cardiovascular outcomes as well as incidence and mean time to Alzheimer’s dementia associated with the long-term use of ginkgo for 5–6 years in approximately 3000 elderly (age 70 or older) adults with normal cognition or mild cognitive impairment. Daily use of 240 mg/d EGb761 did not affect the incidence of hypertension or reduce blood pressure among persons with hypertension or prehypertension. No significant effects in cardiovascular disease mortality, ischemic stroke or events, or hemorrhagic stroke were observed.
2. Metabolic effects—
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


