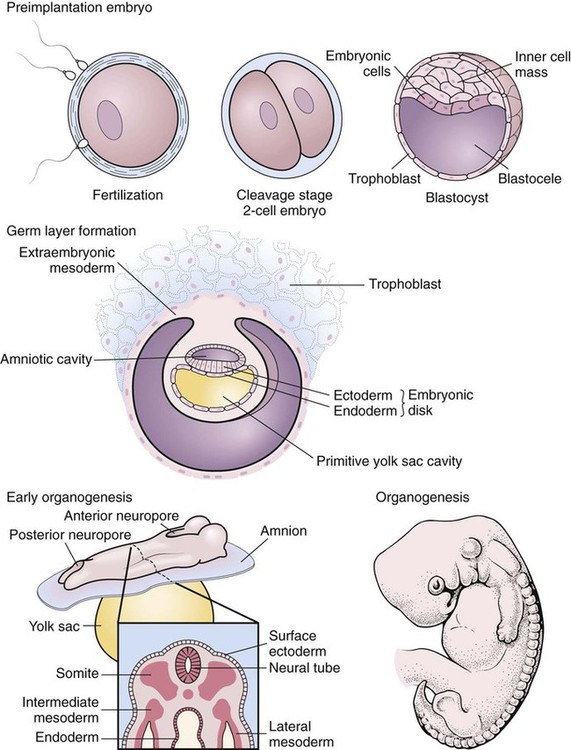
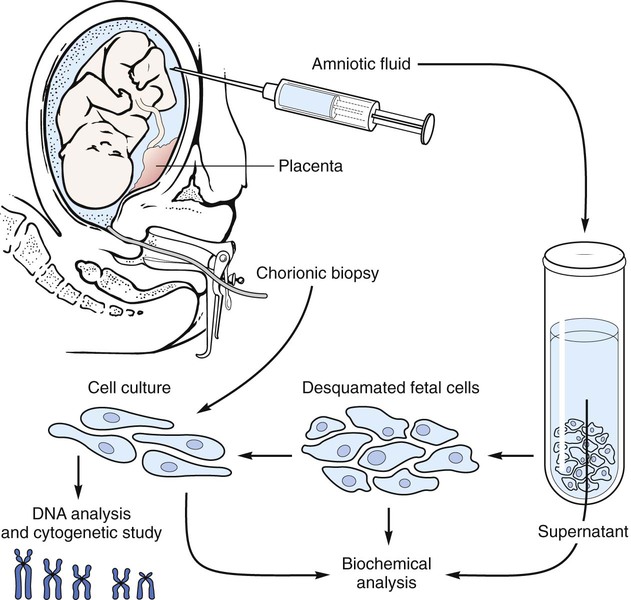
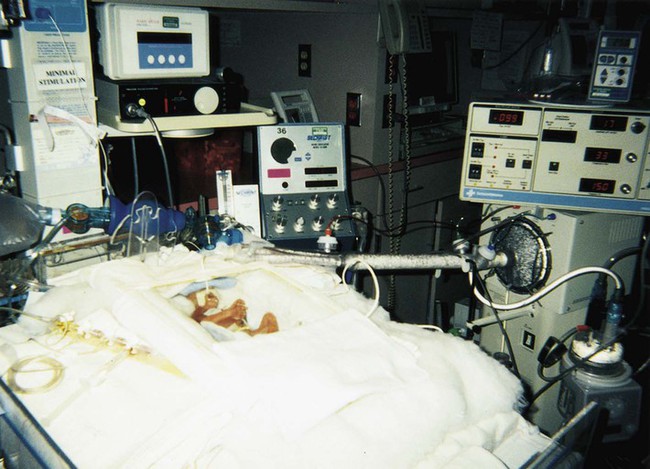
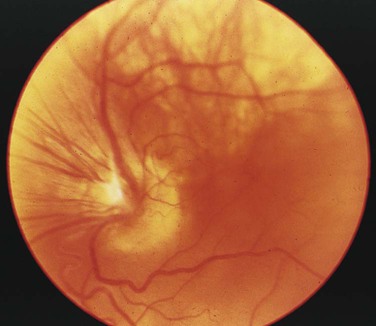
After studying Chapter 2, you should be able to: 1. List the possible causes of congenital anomalies. 2. Discuss the purpose and procedure of amniocentesis. 3. Discuss genetic disorders and syndromes. 5. Describe the condition of prematurity and associated disorders: the causes and treatment. 6. List possible risk factors of retinopathy of prematurity. 7. List symptoms and signs of Down syndrome. 8. List and discuss diseases of the pediatric nervous system. 9. Distinguish between muscular dystrophy and cerebral palsy. 10. Describe patent ductus arteriosus. 11. Name and describe the most common congenital cyanotic cardiac defect. 12. List and discuss musculoskeletal conditions of newborns and young children. 13. List and discuss pediatric genitourinary conditions. 14. List and discuss pediatric diseases of the digestive system. 15. List the major clinical manifestations of cystic fibrosis. 16. Distinguish between Klinefelter’s syndrome and Turner’s syndrome. 17. Describe the clinical condition of congenital rubella syndrome. 18. List and discuss contagious diseases of children. 19. Discuss possible causes and prevention of SIDS. 20. Distinguish between croup and epiglottitis. 21. Describe the symptoms, signs, and treatment for tonsillitis. 22. Discuss the treatment of asthma. 23. List types of worms that may infest the GI tract. 24. List the symptoms and signs of anemia; describe the pathology of leukemia. 25. Explain the etiology of erythroblastosis fetalis. 26. Name some warning signs of lead poisoning. 27. Describe the infant born with fetal alcohol syndrome. The developmental process begins with conception and progresses as a gradual modification of the structure and characteristics of the individual (Figure 2-1). The first 2 months of the gestational period is considered the embryonic period, after which the developing human being is considered a fetus. At any point in this prenatal development, during the birth process (perinatal period), or during the neonatal and postnatal periods, the development may diverge from normal, generating a developmental dilemma. Causes of these dilemmas can be many or even unknown. Pregnant women are encouraged to abstain from smoking, consuming alcohol, and taking any form of medication or drugs without their physician’s knowledge and consent and to prevent any situation that may expose the developing fetus to toxic substances. Table 2-1 describes the specific stages of development during this important period of life. E2-1 TABLE 2–1 Monthly Changes During Prenatal Development *These are 4-week (28-day) months. From Applegate EJ: The anatomy and physiology learning system, ed 3, Philadelphia, 2006, Saunders. (P07.00-P07.03 = 4 codes of specificity) P07.10 (Other low birth weight newborn, unspecified weight) (P07.10-P07.18 = 6 codes of specificity) 765.20 (Unspecified weeks of gestation) 765.21 (Less than 24 completed weeks of gestation) 765.22 (24 completed weeks of gestation) 765.23 (25-26 completed weeks of gestation) 765.24 (27-28 completed weeks of gestation) 765.25 (29-30 completed weeks of gestation) 765.26 (31-32 completed weeks of gestation) 765.27 (33-34 completed weeks of gestation) 765.28 (35-36 completed weeks of gestation) P07.20 (Extreme immaturity of newborn, unspecified weeks) (P07.20-P07.23 = 4 codes of specificity) P07.30 (Other preterm newborn, unspecified weeks) (P07.30-P07.32 = 3 codes of specificity) P07.21 (Extreme immaturity of newborn, less than 24 completed weeks) P07.22 (Extreme immaturity of newborn, 24-26 completed weeks) P07.23 (Extreme immaturity of newborn, 27 completed weeks) P07.31 (Other preterm newborn, 28-31 completed weeks) P07.32 (Other preterm newborn, 32-36 completed weeks) Treatment varies depending on the gestational age, weight, present or subsequent conditions, anomalies, and nutritional status. Intravenous (IV) fluids and hyperalimentation are necessary to encourage growth and development of the premature infant. Airway management and pulmonary functioning are monitored very closely. Many of the smallest babies are intubated endotracheally, and respiration is maintained by mechanical ventilation. Recent advances in respiratory care for tiny premature infants permit extubation of the infant and maintenance of the airway by continuous positive airway pressure (CPAP) through the nose. Pulse oximeters constantly monitor oxygen (O2) saturation levels and heart rate. Body temperature is monitored closely and maintained at normal levels (Figure 2-4). When infection is a risk due to maternal prenatal infection or extended time of ruptured amniotic sac, vigilance is required for the onset of any symptoms of infection and antibiotics are often started presumptively. When early signs of infection occur, aggressive treatment is introduced. Monitoring of blood glucose, blood pressure, and body temperature are performed on a frequent and regular basis and any treatment necessary is instituted to maintain optimal levels. Advances in technology have made survival of low-weight and short-gestation infants possible. The prognosis for these children varies depending on gestational age, weight, and the occurrence of anomalies and developmental deficits. There are documented cases of 12-ounce and/or 22-gestational-week babies surviving. They fall into the 1% of premature babies born at that weight and gestational age. Being born before the normal prenatal development is complete, these children often have many problems to overcome (Figure 2-5). The generally accepted gestational age for 50% of infants to survive the birth process and perinatal period is 24 weeks, with a greater percentage of infants surviving as gestational age increases. Improvements in technology are making it possible for more and more of these tiniest infants to survive (Figure 2-6). E2-5 Preventing prematurity requires good prenatal care, adequate nutrition, and assessment of the pregnant patient’s risk factors for premature labor. Abstaining from consuming alcohol and smoking cigarettes helps to reduce the risk of premature birth and/or low birth weight. Additionally, bed rest of the expectant mother may delay the onset of premature labor and create more time for the fetus to develop. Drug therapy may be used in an attempt to stop premature labor that results in premature birth (see Chapter 12). ROP occurs most often in infants born before 28 weeks of gestation. There are no visible symptoms. Screening examinations are performed routinely on premature infants weighing less than 1500 g or at a gestational age of less than 30 weeks. The entire retina is visualized to determine the stages of development of the blood vessels supplying it. These examinations first are performed when the infant is 4 to 6 weeks old (Figure 2-7).
Developmental, Congenital, and Childhood Diseases and Disorders
Developmental and Congenital Disorders
Developmental Characteristics and Congenital Anomalies
END OF MONTH*
SIZE OF EMBRYO OR FETUS
DEVELOPMENTS DURING THE MONTH
1
6 mm
Arm and leg buds form; heart forms and starts beating; body systems begin to form.
2
23-30 mm, 1 g
Head nearly as large as body; major brain regions present; ossification begins; arms and legs distinct; blood vessels form and cardiovascular system fully functional; liver enlarges.
3
75 mm, 10-45 g
Facial features present; nails develop on fingers and toes; can swallow and digest amniotic fluid; urine starts to form; fetus starts to move; heartbeat detected; external genitalia develop.
4
140 mm, 60-200 g
Facial features well formed; hair appears on head; joints begin to form.
5
190 mm, 250-450 g
Mother feels fetal movement; fetus covered with fine hair called lanugo hair; eyebrows visible; skin coated with vernix caseosa, a cheesy mixture of sebum and dead epidermal cells.
6
220 mm, 500-800 g
Skin reddish because blood in the capillaries is visible; skin wrinkled because it lacks adipose in the subcutaneous tissue.
7
260 mm, 900-1300 g
Eyes open; capable of survival but high mortality rate scrotum develops; testes begin their descent.
8
280-300 mm, 1400-2100 g
Testes descend into the scrotum; sense of taste is present.
9
310-340 mm, 2200-2900 g
Reddish skin fades to pink; nails reach tips of fingers and toes or beyond.
10
350-360 mm, 3000-3400 g
Skin smooth and plump because of adipose in subcutaneous tissue; lanugo hair shed; fetus usually turns to a head-down position; full term.
Prematurity
Preterm Birth or Prematurity
Description
 ICD-9-CM Code 765.00 (Extreme immaturity)
ICD-9-CM Code 765.00 (Extreme immaturity)
 ICD-10-CM Code P07.00 (Extremely low birth weight newborn, unspecified weight)
ICD-10-CM Code P07.00 (Extremely low birth weight newborn, unspecified weight)
 ICD-9-CM Code 765.10 (Other preterm infants)
ICD-9-CM Code 765.10 (Other preterm infants)
 ICD-10-CM Code P07.00 (Extremely low birth weight newborn, unspecified weight)
ICD-10-CM Code P07.00 (Extremely low birth weight newborn, unspecified weight)
Treatment
Prognosis

Prevention
Retinopathy of Prematurity
Symptoms and Signs
Developmental, Congenital, and Childhood Diseases and Disorders