CHAPTER 12 Development of the Spine and Spinal Cord
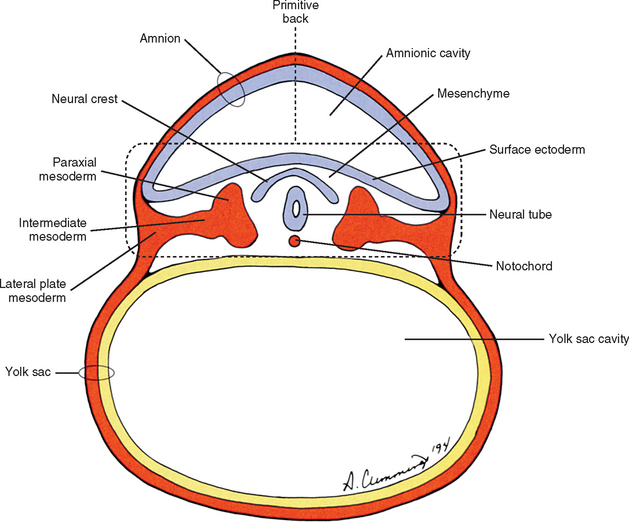
The human back can be identified as early as the end of the third week of development when the embryo consists of a disc of cells between the floor of the amnionic cavity and the roof of the yolk sac cavity (Fig. 12-1). Because the embryo is a flattened disc of cells, no complete body wall (i.e., no sides or front) exists, only the back (Brash, 1951b). Even at this early point, all the primordia that make up the definitive back are present: the surface ectoderm, neural tube, neural crest, notochord, and paraxial mesoderm. The posterior boundary of the back is the surface ectoderm flooring the amnionic cavity, and its anterior boundary is the endoderm roofing the yolk sac cavity.
BLASTOCYST
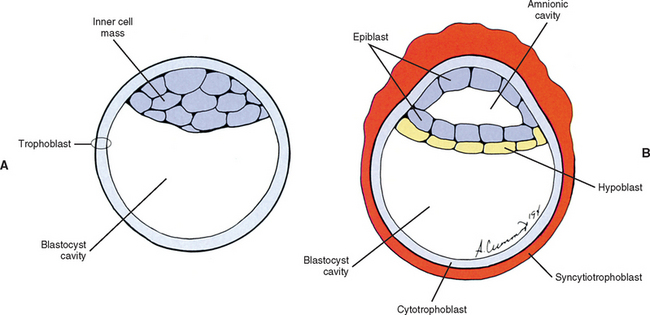
The human begins as the fertilized ovum, which cleaves itself into smaller and smaller embryonic cells known as blastomeres, each carrying all the genetic information needed to build any tissue or organ in the body (Williams et al., 1995). These cells at first form a solid sphere, but rearrangement and continued cell division produce a hollow sphere known as the blastocyst (Patten, 1964). The blastocyst is a single cell layer thick except at one pole, where the cells form into a disc that is two cell layers thick (Brash, 1951b) (Fig. 12-2, A). This disc is called the inner cell mass to distinguish it from all the other cells, which are referred to collectively as the trophoblast (Mossman, 1987). The hole in the center of the blastocyst is known as the blastocyst cavity.
The inner cell mass and the trophoblast have different fates. The former goes on to form the embryo proper. The latter can be likened to embryonic scaffolding that eventually is dismantled or discarded. Before that happens, trophoblast cells proliferate by mitosis until they are several cell layers thick. The innermost layer of the trophoblast still lines the blastocyst cavity. It remains cellular and is called the cytotrophoblast (Baxter, 1953). The cells in the outermost layer begin to lose their cell membranes, thus forming a syncytium known as the syncytiotrophoblast (Williams et al., 1995) (Fig. 12-2, B). This syncytium releases proteolytic enzymes that enable the blastocyst to digest its way into the lining of the uterus, called the endometrium. This process is known as implantation and usually is completed by the end of the second embryonic week. The implanted blastocyst does not leave the uterine wall until it bursts out in the form of a newborn (Beck et al., 1985).
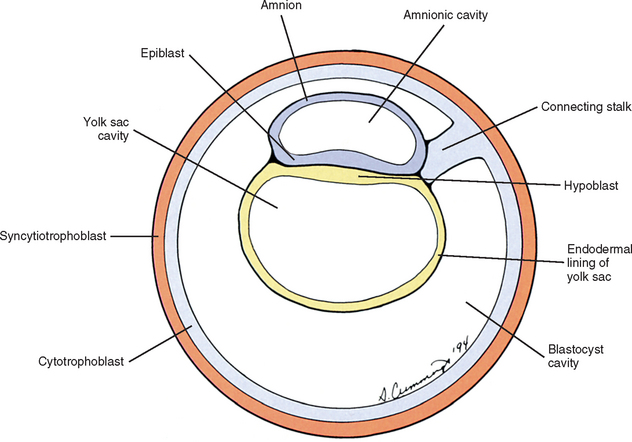
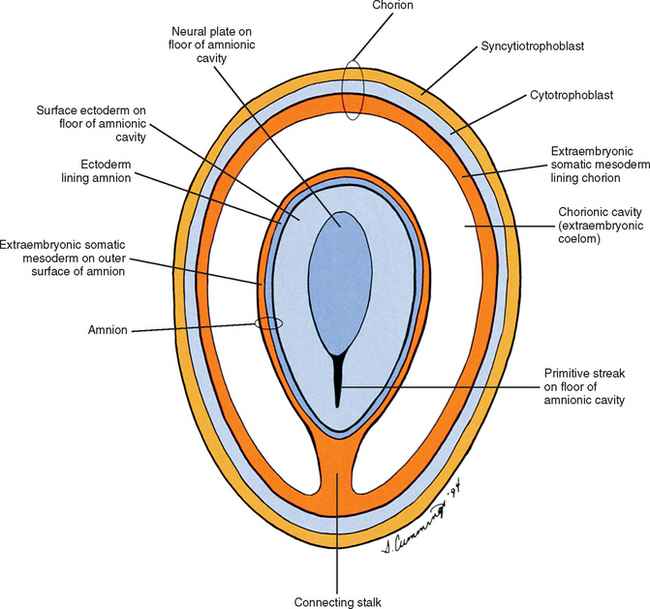
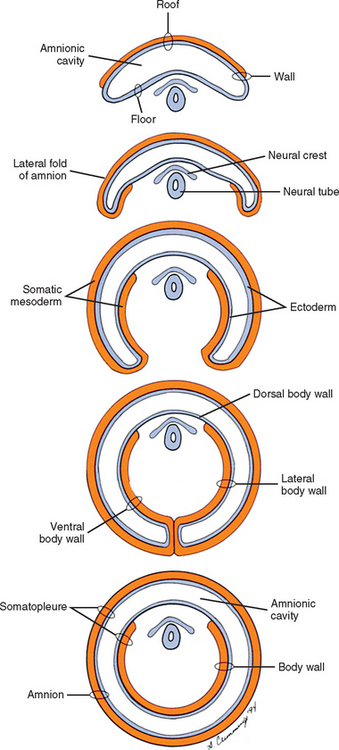
Also during the second week of development, the inner cell mass has been changing (Williams et al., 1995). A space, the first sign of the amnionic (amniotic) cavity, has appeared within it (see Fig. 12-2, B). The amnionic cavity can be thought of as being enclosed by walls, a roof, and a floor (Langebartel, 1977). The roof and walls become the amnion. The floor becomes the dorsal surface of the embryo and later transforms into the outer layer of the skin on the newborn’s back (Sadler, 2004). By this time the inner cell mass is attached to the cytotrophoblast by only a narrow bridge of cells that is called the connecting stalk (Fig. 12-3). The connecting stalk marks the tail or caudal end of the embryo just as the amnion marks its dorsal surface. A close observer would note that the cells in the inner cell mass have grouped themselves into two layers (see Fig. 12-2, A). One layer floors the amnionic cavity and is called the epiblast. The other layer roofs the blastocyst cavity and is called the hypoblast (Moore and Persaud, 2003). The hypoblast gives rise to a layer of cells that comes to line the blastocyst cavity. The blastocyst cavity then is known as the primitive yolk sac even though it contains a negligible amount of yolk (Scammon, 1953) (see Fig. 12-3).
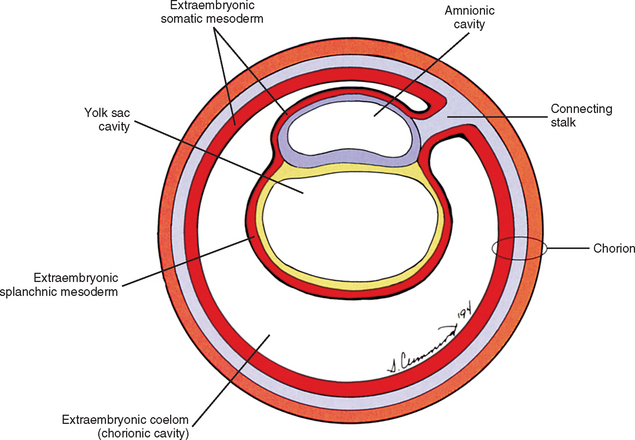
The yolk sac cells next bud off other cells that deposit themselves on the outside of the yolk sac, where they make up a layer called extraembryonic mesoderm (Moore and Persaud, 2003) (Fig. 12-4). The triple layer, consisting of the cytotrophoblast sandwiched between the syncytiotrophoblast and extraembryonic mesoderm, is called the chorion and is the embryonic portion of the placenta (Goss, 1966). That means the cavity inside the chorion actually corresponds to the previous blastocyst cavity or primitive yolk sac (Sadler, 2004). To recognize the changes that have occurred (e.g., the imminent appearance of the secondary or definitive yolk sac and extraembryonic mesoderm), this cavity is renamed the extraembryonic coelom and is also called the chorionic cavity (Goss, 1966) (see Fig. 12-4).
GASTRULATION AND DEVELOPMENT OF THE NOTOCHORD
At the beginning of the third week of development, a narrow groove forms on the caudal portion of the dorsal surface of the epiblast known as the primitive streak (Hamilton and Mossman, 1972) (Fig. 12-5). Epiblast cells begin to migrate toward the primitive streak. Once there, the cells detach from the epiblast and invaginate deep to it through the primitive streak. Once invaginated, the cells move in all directions. Some of these cells displace the hypoblast and form the endoderm (Sadler, 2004). The endoderm eventually will form the lining of the respiratory and gastrointestinal tracts. Other invaginating cells form a new layer of cells between the epiblast and the newly formed endoderm called the intraembryonic mesoderm. The cells remaining in the epiblast become the ectoderm. The ectoderm, mesoderm, and endoderm are the three germ layers from which the entire embryo will differentiate. The process of forming these germ layers and creating a trilaminar embryonic disc is known as gastrulation.
Some of the invaginated epiblast cells moving superiorly in the midline toward the future head turn into a structure known as the notochord (Fig. 12-6; see also Fig. 12-1). The notochord is a relatively rigid rod of cells that marks the longitudinal axis of the embryonic body and induces the nervous system to develop. The vertebral column later occupies this site, and by then the notochord has turned into the part of the intervertebral disc known as the nucleus pulposus.
NEURULATION
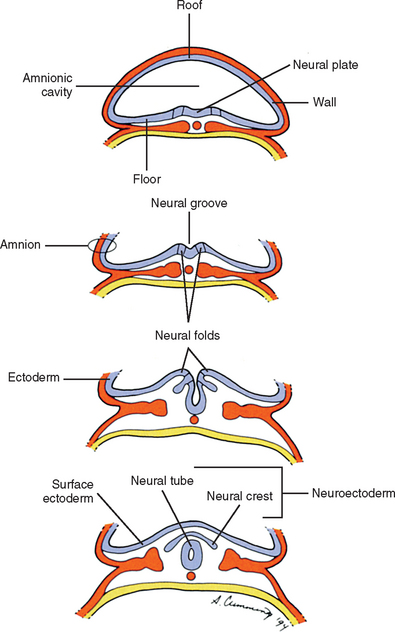
Also during the third week of development, the notochord induces the ectoderm to form a thickening along its length in the midline called the neural plate, which is the primordium of the entire nervous system (Arey, 1965). The cells of the neural plate are known as the neuroectoderm. A groove forms, which runs the length of the neural plate, with two folds flanking it. This neural groove deepens and sinks below the surface. The neural folds close over it, thus forming a hollow tube of neuroectoderm called the neural tube (Scothorne, 1976) (Fig. 12-7). The neural tube is the primordium of the brain and spinal cord, and the process of its formation is called neurulation. Other neuroectoderm cells invaginate along with the neural tube but remain apart from it (Romanes, 1972a,b). They are collectively known as the neural crest (see Fig. 12-7) and give rise, among other things, to many important components of the nervous system, including all sensory neurons, all postganglionic autonomic neurons, and all ganglia, both sensory and motor (Scothorne, 1976). The rest of the ectodermal cells, which are not part of the neural plate, are destined mostly to form the epidermis (the surface layer of the skin), and are appropriately named surface ectoderm.
SOMITE DEVELOPMENT
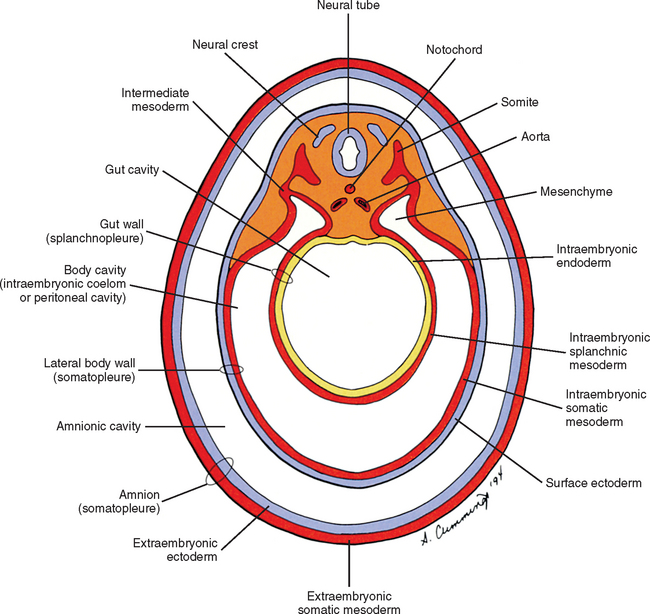
The mesoderm lying just lateral to the notochord parallels the long axis of the embryo and is called the paraxial mesoderm. Beginning during the third embryonic week, the paraxial mesoderm subdivides into blocks of cells known as somites, which are major contributors to the muscles, bones, and the dermis of the body. Just lateral to the somites on both sides of the embryo, other mesodermal cells have formed into a structure called intermediate mesoderm. The intermediate mesoderm eventually will develop into the majority of the genitourinary system (Fig. 12-8; see also Fig. 12-1). Lateral to the intermediate mesoderm is the lateral mesoderm, which will, along with the overlying ectoderm, form the lateral and ventral parts of the body wall.
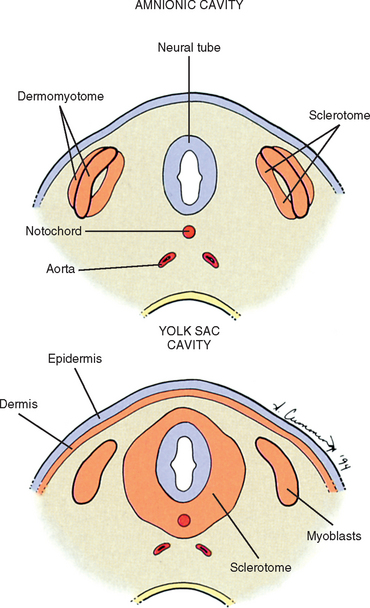
The somites are staging areas from which mesoderm cells deploy to new locations (Sadler, 2004). The cells of the ventromedial portion of the somite will migrate toward the notochord and neural tube. These cells eventually produce hard tissues, such as bone, cartilage, and ligaments, so the part of the somite from which they are derived is appropriately called sclerotome (Harrison, 1972) (Fig. 12-9).
The rest of the somite is called the dermomyotome or epithelial plate of the somite. Traditionally, once myoblasts could be identified in the somite, termed the myotome, the remainder of the somite was called the dermatome, which went on to form the dermis of the skin (Beck et al., 1985) (see Fig. 12-9). It is now believed that the superficial portion of the somite, the region of the traditionally defined dermatome, contributes a significant amount of muscle precursor cells as it elongates through the body wall (Williams et al., 1995). Also, the only dermis that appears to be derived exclusively from somites may be that covering the epaxial musculature, which is a much more restricted distribution than implied by the term dermatome. The remainder of the dermomyotome forms myoblasts that will eventually form skeletal muscles (Fischman, 1972) (see the following).
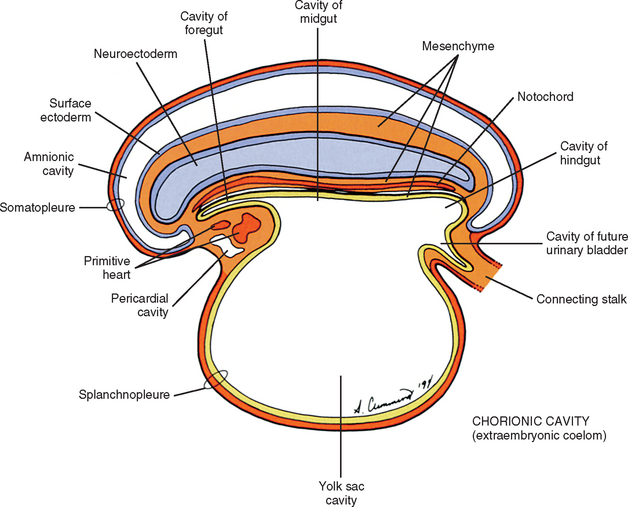
During the next several weeks, the amnion undergoes a series of folds creating the embryo’s body wall and defining the lumen of the embryonic gut (Fig. 12-10). The embryo’s body cut in cross section at this time appears as two concentric rings. The inner ring is the splanchnopleure; the outer is the somatopleure (Goss, 1966) (see Fig. 12-8). The outer ring actually has the shape of a jeweler’s signet ring. Tucked close together inside the signet part are the neural tube, neural crest, notochord, somites, intermediate mesoderm, and mesenchyme (see Fig. 12-8). The signet part corresponds to what is variously called the back, the posterior body wall, and the posterior abdominal or posterior thoracic wall (Callander, 1939; Grant, 1952; Davenport, 1966).
VERTEBRAL DEVELOPMENT
Typical Vertebrae
Vertebral development can be conveniently divided into four stages. The notochordal stage is first. The notochord not only forms the original basis for the development of the vertebrae by defining the axis of the embryo, it also forms some of the cells of the intervertebral disc. The second stage is the mesenchymal, or blastemal, stage. Mesenchyme is the connective tissue of the embryo that is, at least in the case of the vertebrae, derived from paraxial mesoderm. Each vertebra is formed from a mesenchymal model. The third stage is the cartilage stage, in which the vertebral mesenchyme is replaced by hyaline cartilage. The osseous stage is last, in which the hyaline cartilage is replaced by bone. Therefore each vertebra is formed by the process of endochondral ossification.
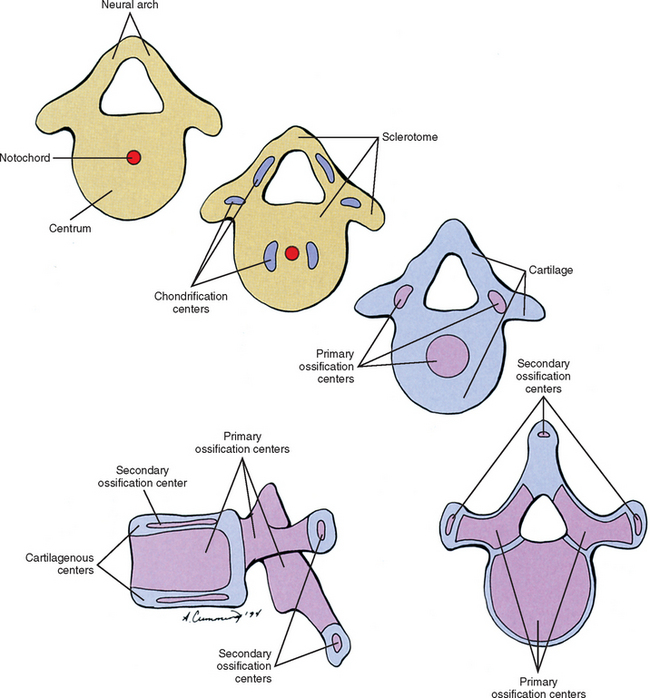
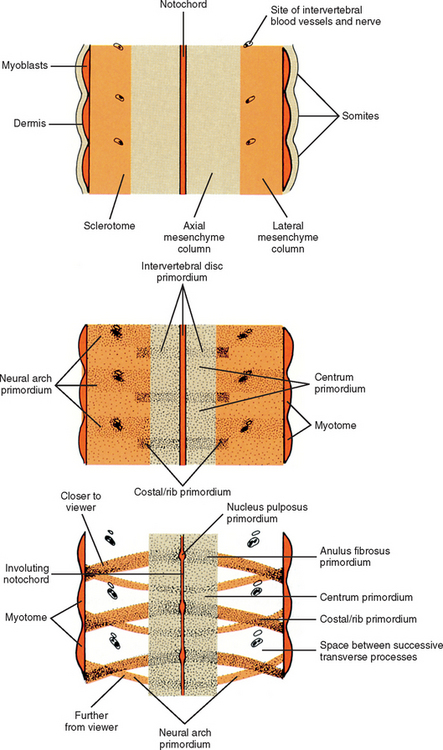
Cells of the sclerotome migrate medially from the somite to surround the neural tube and notochord (Breathnach, 1958). Instead of forming a continuous tunnel of cells, they form a series of discrete blocks of mesenchyme (Williams et al., 1995). Those cells ventral to the neural tube that specifically surround the notochord are known as the centrum. The cells dorsolateral to the neural tube are called the neural arch (O’Rahilly, 1986) (Fig. 12-11). Each neural arch forms a pedicle, which projects ventromedially toward the centrum, and a lamina, which projects dorsomedially toward the corresponding lamina from the other side. The laminae eventually fuse and expand to form the spinous process. Cranial and caudal projections of the neural arch form articular processes, which will develop into the zygapophysial joints with adjacent vertebrae. A lateral projection of the neural arch forms the true transverse process. Projecting anterolaterally from the neural arch is the costal element, which expands to meet the tip of the transverse process. The patterning of the mesenchyme into presumptive vertebrae appears to be regulated by HOX genes (Sadler, 2004).
For years the accepted view has been that the sclerotome cells forming a centrum derived from two contiguous somites (Beck et al., 1985). This dual origin accounts for the staggered position of myotomes and centra (Sadler, 2004). This accepted view was challenged by Verbout (1985). However, experimental support for the accepted view also has been adduced (Bagnall et al., 1987). Most evidence still suggests that approximately the cranial half of a centrum is derived from the caudal portion of the somite above, whereas the caudal half of a centrum is derived from the cranial portion of the somite below (Williams, 1995) (Fig. 12-12).
Regardless of their origin, by the end of the second embryonic month, some of the mesenchymal cells of the vertebrae change into chondroblasts and begin laying down an extracellular cartilaginous matrix (Hall, 1978). There are typically two chondrification centers in each centrum, located on either side of the notochord. These rapidly replace the notochord in the region of the centrum with cartilage to form a single mass of cartilage (Williams et al., 1995) (see Fig. 12-11). Also, each side of the neural arch develops at least one chondrification center.
By the end of the embryonic period, primary ossification centers appear in the cartilaginous vertebra. These usually appear in a cranial to caudal pattern, beginning in the upper cervical region by approximately week 8 and reaching the sacral area by week 22 (Williams, 1995). Typically two primary ossifications centers form in the centrum (ventral and dorsal), which rapidly fuse into one center (Moore and Persaud, 2003). There is also a primary ossification center that develops in each side of the neural arch (O’Rahilly & Muller, 1992) (see Fig. 12-11). By birth, each vertebra consists of three bony segments connected by hyaline cartilage: the centrum and each half of the neural arch. The cartilages between the centrum and each neural arch actually are located within the region that will become the adult vertebral body. This region is known as the neurocentral synchondrosis (or junction) and usually fuses together into compact bone by age six (Maat et al., 1996). Therefore the terms vertebral body and centrum are not equivalent. Likewise the terms neural arch and vertebral arch are not synonymous. The vertebral body encompasses all of the centrum and a portion of each neural arch. The vertebral arch is only a portion of each neural arch (Trotter and Peterson, 1966).
At approximately the time of puberty, secondary ossification centers appear in the remaining cartilage (Sinclair, 1972) (see Fig. 12-11). Typically each vertebra develops five secondary ossification centers: one for the tip of each transverse process, one for the tip of the spinous process, and two anular apophyses (outdated term: anular epophyses) one on the superior and one on the inferior rim of the vertebral body. A vertebra can increase in height and diameter before and after birth by employing intrinsic bone-depositing mechanisms around the circumference, or periosteum, and at the ends (Bogduk and Twomey, 1987). Also, there can be some variation in the distribution, and sometimes even number, of ossification centers from vertebra to vertebra, accounting for some of their individual variation (Inkster, 1951).
Although each vertebra has costal elements, the eventual fates of these elements vary by region of the spine. In the cervical spine, the costal elements eventually form most of the adult transverse process, including the portions that form the ventral and dorsolateral boundaries of the foramen of the transverse process. Only the portion of the adult transverse process that forms the dorsomedial border of the foramen of the transverse process develops from the embryonic transverse process. In the thoracic region, the costal elements reach their maximal length and form the ribs. In the lumbar spine, the costal elements form the anterior and lateral portions of the adult transverse processes. The embryonic transverse process forms only the dorsomedial portion of the adult lumbar transverse process, which includes the accessory process (Robertson, 1966).
Atypical Vertebrae (Developmentally)
Atlas.
In the mesenchyme stage, most of the cells of the posterior aspect of the centrum of the first cervical vertebra actually become associated with the superior aspect of the cells of the centrum of the second cervical vertebra. This detachment of cells accounts for the lack of a vertebral body in the atlas and gives this bone its characteristic ring shape. These “extra” cells will become the odontoid process of the axis. The remaining cells of the anterior aspect of the centrum of the atlas will become the anterior arch of that bone. The atlas usually is ossified from three ossification centers. There is one ossification center for each lateral mass. Also, a third ossification center forms for the anterior arch, but this center does not usually develop until after the time of birth (Pharaoh, 1956).
Axis.
Most of the mesenchyme cells from the posterior aspect of the centrum of the atlas become associated with the superior aspect of the centrum of the axis. These cells will eventually become the odontoid process, although there is recent evidence that the odontoid process is not derived solely from the centrum of the atlas (David et al., 1998). The axis usually has five primary and two secondary ossification centers. The centrum and neural arches ossify in the typical manner (one center for each). At the beginning of the third trimester of pregnancy, two ossification centers appear laterally in the base of the cartilaginous odontoid process. A thin piece of cartilage may separate the base of the odontoid process and the body of the axis. This rudimentary disc ossifies slowly and may not completely disappear until the latter teenage years. A secondary ossification center forms in the apex of the odontoid process at approximately age 3 to 6 and unites with the rest of the odontoid process by approximately age 12. The last secondary ossification center is a typical anular apophysis associated with the inferior aspect of the body of the axis.
Seventh Cervical.
The seventh cervical vertebra has the typical ossification centers with addition of separate secondary ossification centers for the costal processes. These appear at approximately the sixth fetal month and unite with the rest of the vertebra at approximately age 6. These may remain separate from the rest of the vertebra and form cervical ribs. Occasionally separate ossification centers for the costal processes can be found for the sixth, fifth, and even fourth cervical vertebrae (Williams et al., 1995).
Sacrum.
The sacrum begins as five vertebral segments that eventually fuse into a single bone. Primary ossification centers for the centrum and neural arches of each sacral vertebra appear between the tenth and twelfth weeks of development. The neural arches and centra begin uniting during the second year. This process begins in the lower segments and is completed in the upper segments by the sixth year. The neural arches from both sides are usually fused by approximately the eighth year. Primary ossification centers also appear for the costal elements of the upper three (and sometimes more) segments superior and lateral to the pelvic sacral foramina during the third trimester of pregnancy. The costal elements fuse with the corresponding neural arches during the second to fifth years and to the centra by approximately the eighth year. Laterally the conjoined neural arches and costal elements are separated from the segments above and below by epiphyseal cartilages. These epiphyseal cartilages are associated with the auricular surfaces. Around the time of puberty, various secondary ossification centers may form that are associated with the upper and lower aspects of the centra, spinous tubercles, transverse tubercles, and costal elements. The centers for the costal elements complete the ossification process for the auricular surfaces by age 20 (Pharaoh, 1956).
The bodies of the sacral vertebrae during early life are separated by intervertebral fibrocartilages. The margins of the bodies begin to fuse around age 18, beginning inferiorly. By the mid-twenties, the sacrum usually is a single, solid bone, although vestiges of the intervertebral fibrocartilages may persist (Williams et al., 1995).
Coccyx.
Each segment of the coccyx ossifies from a single primary ossification center. The center for the first segment appears soon after the time of birth. Occasionally there are separate ossification centers that appear for the coccygeal cornua at approximately this same time (Williams et al., 1995). The ossification centers for the remaining coccygeal segments appear, from superior to inferior, variably during childhood and puberty, so that all of them have usually appeared by age 20. The segments gradually fuse with one another so that by age 30 the coccyx usually is a solid bone. The coccyx may variably fuse with the sacrum in later life.
Intervertebral Discs and Other Joints
Although the mesenchyme of the sclerotome replaces the notochordal tissue to form the centra of the vertebrae, the notochordal tissue located between the developing vertebrae actually expands to form the presumptive nucleus pulposus of the intervertebral discs (see Figs. 12-9 and 12-12
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree