Fig. 14.1
Comparison of desmoplastic nevus (left) and desmoplastic melanoma (right). Desmoplastic nevus, as some Spitz nevi do, may show many cells expressing HMB45 antigen in the dermis; however, anti-Ki67 shows very rare dermal cells. In contrast, desmoplastic melanoma is usually negative with HMB45 and shows numerous cells expressing Ki67, usually more than 20/mm2 (H) (HMB-45 and anti-Ki67, aminoethylcarbazol and diaminobencidine, respectively, with light hematoxylin as counterstain)
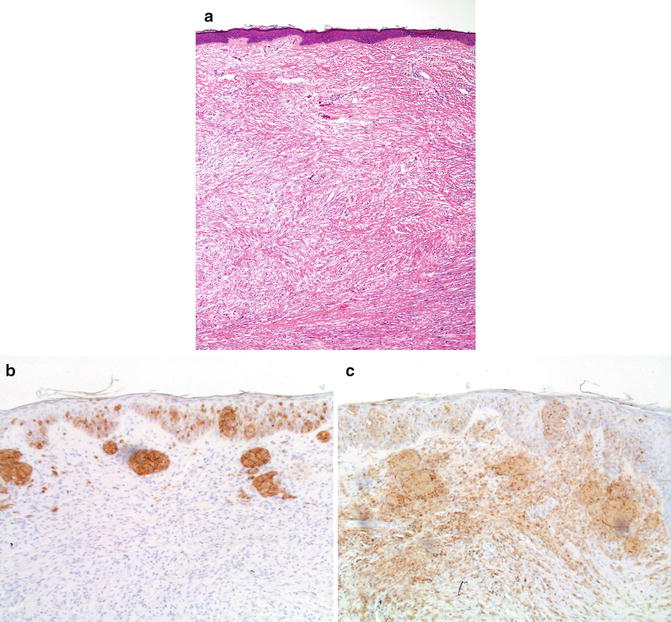
Fig. 14.2
Desmoplastic melanoma Illustration of the use of anti-MART1 in the differential diagnosis of desmoplastic lesions. Flat epidermis with hyperchromatic cells in the dermis (a). Only intraepidermal cells and very rare, superficial dermal cells express MART1 (b). In contrast, both the intraepidermal and dermal components are strongly positive for S100 protein (c) (a: hematoxylin and eosin; b: anti-MART1; c: anti-S-100 protein; both b and c, aminoethylcarbazol, with light hematoxylin and eosin)
To distinguish between desmoplastic melanoma and malignant peripheral nerve sheath tumor, S-100 protein is uniformly positive in melanoma and it is usually patchy or negative in malignant peripheral nerve sheath tumor. MITF is focally positive in both lesions. All other melanocytic markers are uniformly negative in both lesions.
Also, it has been proposed that immunohistochemical labeling for p16 may be helpful in such distinction, as the majority of desmoplastic melanomas show loss of labeling with p16. In our opinion, there is significant overlap between nevi and melanoma regarding p16 expression, also seen in Spitz lesions [10]; therefore we do not consider p16 to be a very useful marker in this differential diagnosis.
Immunohistochemistry is also helpful in the diagnosis of desmoplastic melanoma and scar, since the tumor cells will express S100 protein. Even though scar tissue has scattered S100-positive cells [11, 12] (Fig. 14.3), when compared with scars, desmoplastic melanomas will have many more cells positive for this marker. Other markers expressed in desmoplastic melanoma and not in scars are p75 [13] and SOX10 [14] (Fig. 14.4). The latter appears to be a very promising marker since in our experience the vast majority of melanomas express this marker, regardless of the histologic subtype.
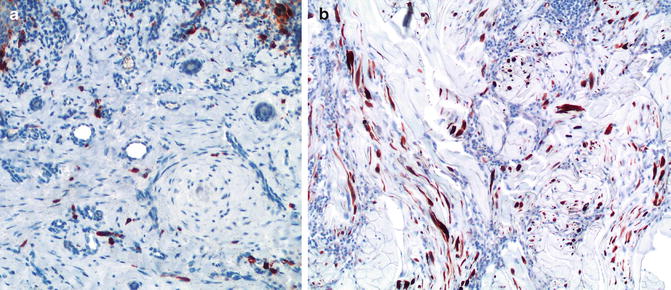
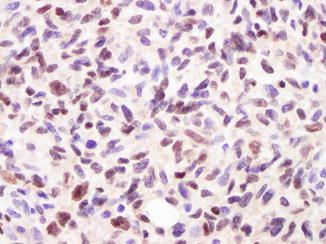

Fig. 14.3
In contrast with dermal scars (a), desmoplastic melanoma (b) shows numerous S100-positive cells (dendritic cells) (anti-S100; diaminobencidine and light hematoxylin as counterstain)

Fig. 14.4
Strong expression of SOX10 by many melanoma cells in this lesion (anti-SOX10; diaminobencidine and light hematoxylin as counterstain)
A recent study using fluorescence in situ hybridization (FISH) targeting RREB1, MYB, Cep6, and CCND1 showed that 47 % of desmoplastic melanoma contained detectable aberrations in the probe sites by FISH, while none of sclerosing melanocytic nevi (including sclerosing blue nevi) showed any [15]. Thus a positive FISH result strongly supports the diagnosis of desmoplastic melanoma and makes a diagnosis of a sclerosing melanocytic nevus unlikely.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


