Dermatological Pharmacology
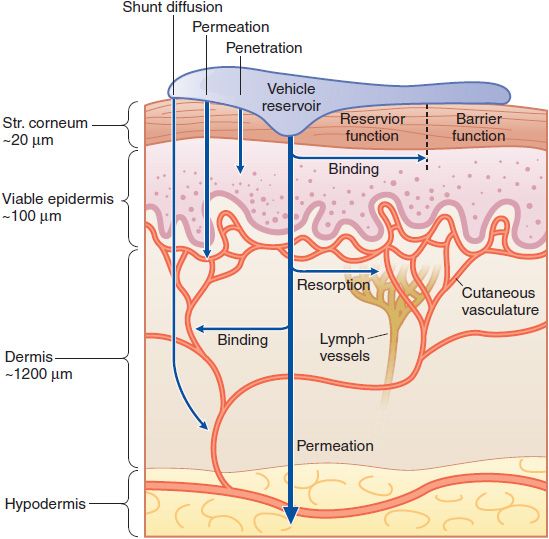
The skin is a multifunctional and multicompartment organ. Figure 65–1 outlines general features of skin structure and percutaneous absorption pathways. Drugs can be applied to skin for 2 purposes: to directly treat disorders of the skin and to deliver drugs to other tissues.
Figure 65–1 Cutaneous drug delivery. Diagrammatic representation of the 3 compartments of the skin as they relate to drug delivery: surface, stratum (Str.), and viable tissues. After application of a drug to the surface, evaporation and structural/compositional alterations occur that determine the drug’s bioavailability. The stratum corneum limits diffusion of compounds into the viable skin and body. After absorption, compounds either bind targets in viable tissues or diffuse within the viable tissue or into the cutaneous vasculature, and thence to internal cells and organs. (Reproduced with permission from Wolff K, et al., eds. Fitzpatrick’s Dermatology in General Medicine, 7th ed. New York: McGraw-Hill; 2008, Figure 215–1. Copyright © 2008 by The McGraw-Hill Companies, Inc. All rights reserved.)
Non-pharmacological therapy for skin diseases includes the entire electromagnetic spectrum applied by many sources, such as lasers, X-rays, visible light, and infrared light. These approaches may be used alone or to enhance the penetration or alter the nature of drugs and prodrugs. Freezing and ultrasound are other physical therapies that alter epidermal structure for direct treatment or to enhance percutaneous absorption of drugs. Chemicals are used to decrease the effect of various wavelengths of ultraviolet (UV) light and ionizing radiation.
Stratum Corneum. The stratum corneum (outer 5-600 μm) is the major barrier to percutaneous absorption of drugs and to the loss of water from the body. Many drugs may partition into the stratum corneum and form a reservoir that will diffuse into the rest of skin even after topical application of the drug has ceased. The stratum corneum differs in thickness, with the palm and sole being the thickest (400-600 μm) followed by the general body stratum corneum (10-16 μm), and the scrotum (5 μm). Facial and post-auricular regions have the thinnest stratum corneum.
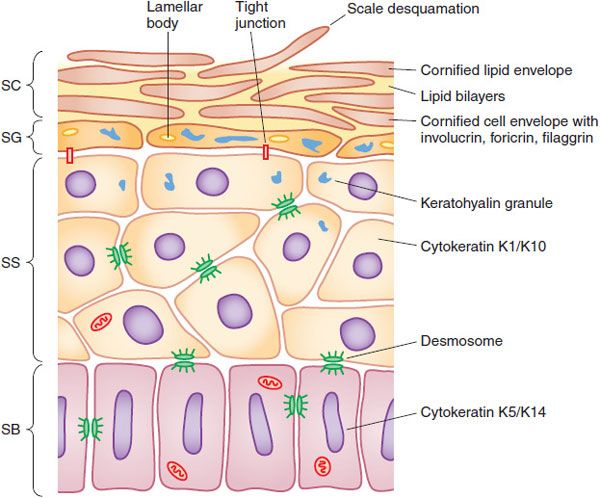
Living Epidermis. The living layers of the epidermis with metabolically active cells comprise a layer ~100 thick (Figure 65–2). Intercalated in the living epidermis are pigment-producing cells (melanocytes), dendritic antigen-presenting cells (Langerhans cells), and other immune cells (γ-δ T-cells); in diseased epidermis, many immunological cells, including lymphocytes and polymorphonuclear leucocytes, may be present and be directly affected by applied drugs.
Figure 65–2 Structure of the epidermis. The epidermis matures progressively from the stratum basale (SB) to the stratum spinosum (SS), stratum granulosum (SG), and stratum corneum (SC). Important structural and metabolic proteins are produced at specific layers of the epidermis. (Reproduced with permission from Wolff K, et al., eds. Fitzpatrick’s Dermatology in General Medicine, 7th ed. New York: McGraw-Hill; 2008, Figure 45–2. Copyright © 2008 by The McGraw-Hill Companies, Inc. All rights reserved.)
Dermis and Its Blood Vessels. The superficial capillary plexus between the epidermis and dermis is the site of the majority of the systemic absorption of cutaneous drugs (see Figure 65–1). There are large numbers of lymphatics as well. Beneath the 1.2-mm-thick dermis with its collagen and proteoglycans that may bind drugs there, targets for drugs include mast cells (permanent residents and producers of many inflammatory mediators) and infiltrating immune cells producing cytokines. Hair follicles form a lipid-rich pathway for drug absorption. Sweat glands are not known as a pathway for the absorption of drugs. Some drugs (e.g., griseofulvin, ketoconazole) are excreted to the skin by this route.
MECHANISMS OF PERCUTANEOUS ABSORPTION. Passage through the outermost layer is the rate-limiting step for percutaneous absorption. Preferable characteristics of topical drugs include low molecular mass (<600 Da), adequate solubility in both oil and water, and a high partition coefficient so the drug will selectively partition from the vehicle to the stratum corneum. Except for very small particles, water-soluble ions and polar molecules do not penetrate significantly through intact stratum corneum. The exact amount of drug entering or leaving the skin in clinical situations usually is not measured; rather, the clinical endpoint (e.g., reduction in inflammation) usually is the desired effect.
A hydrated stratum corneum allows more percutaneous absorption and often is achieved through the selection of drugs formulated in occlusion vehicles such as ointments and the use of plastic films, wraps, or bags for the hands and feet and shower or bathing caps for the scalp, or through the use of medications that are impregnated on patches or tapes. Occlusion may be associated with increased growth of bacteria with resultant infection (folliculitis) or maceration and breakdown of the epidermis. Transport of most drugs is a passive thermodynamic process, and heat generally increases penetration. Ultrasonic energy or laser-induced vibration also can be used to increase percutaneous absorption. The latter may function by the production of lacunae in the stratum corneum.
The epidermis contains a variety of enzyme systems capable of metabolizing drugs that reach this compartment. A specific CYP isoform, CYP26A1, metabolizes retinoic acid and may control its level in the skin. In addition, transporter proteins that influence influx (OATP) or efflux (MDR, P-glycoprotein) of certain xenobiotics are present in human keratinocytes. Genetic variants of enzymes that regulate the cellular influx and efflux of methotrexate have been associated with toxicity and effectiveness in patients with psoriasis.
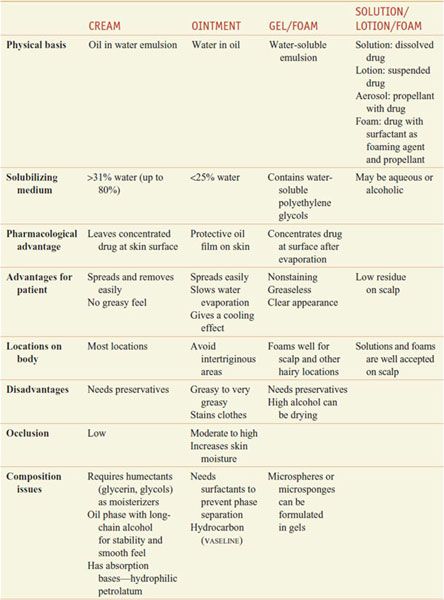
PHARMACOLOGIC IMPLICATIONS OF EPIDERMAL STRUCTURE. The healthcare provider, when proposing topical application of drugs (Table 65–1), must consider proper dosage and frequency of application, extent and condition of the permeability barrier, patient age and weight, physical form of the preparation to be applied, and whether intralesional or systemic administration should be used. Various drug vehicles have specific advantages and disadvantages (Table 65–2). Newer vehicles include liposomes and microgel formulations that can enhance solubilization of certain drugs, thereby enhancing topical penetration and diminishing irritancy. Children have a greater ratio of surface area to mass than adults do, so the same amount of topical drug can result in a greater systemic exposure.
Table 65–1
Important Considerations When a Drug Is Applied to the Skin
Table 65–2
Vehicles for Topically Applied Drugs
GLUCOCORTICOIDS
Glucocorticoids have immunosuppressive and anti-inflammatory properties. They are administered locally, through topical and intralesional routes, and systemically, through intramuscular, intravenous, and oral routes. Mechanisms of glucocorticoid action are discussed in Chapter 42.
TOPICAL GLUCOCORTICOIDS. Topical glucocorticoids have been grouped into 7 classes in order of decreasing potency (Table 65–3).
Table 65–3
Potency of Selected Topical Glucocorticoids
Therapeutic Uses. Many inflammatory skin diseases respond to topical or intralesional administration of glucocorticoids. Absorption varies among body areas; the steroid is selected on the basis of its potency, the site of involvement, and the severity of the skin disease. Often, a more potent steroid is used initially, followed by a less potent agent. Twice-daily application of topical glucocorticoids is sufficient, and more frequent application does not improve response. In general, only nonfluorinated glucocorticoids should be used on the face or in occluded areas such as the axillae or groin. Intralesional preparations of glucocorticoids include insoluble preparations of triamcinolone acetonide (KENALOG-10) and triamcinolone hexacetonide (ARISTOSPAN), which solubilize gradually and therefore have a prolonged duration of action.
Toxicity. Chronic use of class 1 topical glucocorticoids can cause skin atrophy, striae, telangiectasias, purpura, and acneiform eruptions. Because perioral dermatitis and rosacea can develop after the use of fluorinated compounds on the face, they should not be used on this site. Occlusion increases the risk of HPA suppression.
SYSTEMIC GLUCOCORTICOIDS. Systemic glucocorticoid therapy is used for severe dermatological illnesses and generally reserved for allergic contact dermatitis to plants (e.g., poison ivy) and for life-threatening vesiculobullous dermatoses such as pemphigus vulgaris and bullous pemphigoid. Chronic administration of oral glucocorticoids is problematic, given the side effects associated with their long-term use (see Chapter 42).
Daily morning dosing with prednisone generally is preferred, although divided doses occasionally are used to enhance efficacy. Fewer side effects are seen with alternate-day dosing; if chronic therapy is required, prednisone usually is tapered to every other day as soon as practical. Pulse therapy using large intravenous doses of methylprednisolone sodium succinate (SOLU-MEDROL, others) is an option for severe resistant pyoderma gangrenosum, pemphigus vulgaris, systemic lupus erythematosus with multi-system disease, and dermatomyositis. The dose usually is 0.5-1 g given over 2-3 h. More rapid infusion has been associated with increased rates of hypotension, electrolyte shifts, and cardiac arrhythmias.
Toxicity and Monitoring. Oral glucocorticoids have numerous systemic effects, as discussed in Chapter 42. Most side effects are dose-dependent.
RETINOIDS
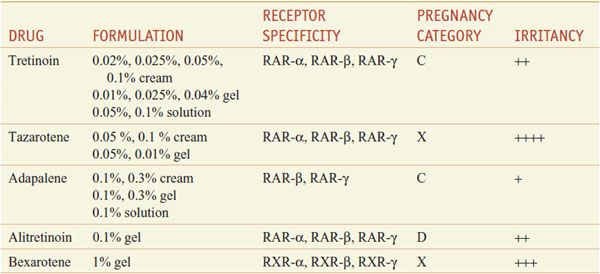
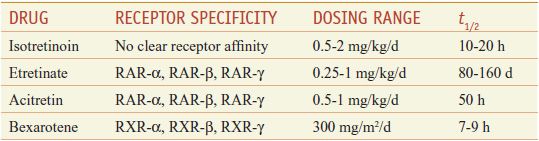
Retinoids comprise natural and synthetic compounds that exhibit vitamin A–like biological activity or bind to nuclear receptors for retinoids (see Figures 3–12 and 6–8). Characteristics of topical and systemic retinoids are summarized in Tables 65–4 and 65–5. Systemic retinoids are used to treat acne and disorders of keratinization.
Table 65–4
Topical Retinoids
Table 65–5
Systemic Retinoids
First-generation retinoids include retinol (vitamin A), tretinoin (all-trans-retinoic acid; vitamin A acid), isotretinoin (13-cis-retinoic acid), and alitretinoin (9-cis-retinoic acid). Second-generation retinoids, also known as aromatic retinoids, include acitretin and methoxsalen (also known as etretinate; not marketed in the U.S.). Third-generation retinoids were designed to optimize receptor-selective binding and include tazarotene, bexarotene, and adapalene.
Mechanism of Action. Retinoids exert their effects on gene expression by activating 2 families of receptors—retinoic acid receptors (RARs) and retinoid X receptors (RXRs)—that are members of the steroid receptor superfamily. Upon binding to a retinoid, RARs and RXRs form heterodimers (RAR-RXR), which subsequently bind specific DNA sequences called retinoic acid–responsive elements (RAREs) that activate transcription of genes whose products produce the desirable pharmacological effects of these drugs and their unwanted side effects (see Table 6–5 and Figures 3–12 and 6–8).
Targeted Therapeutic Actions. Retinoids that target RARs predominantly affect cellular differentiation and proliferation; retinoids that target RXRs predominantly induce apoptosis. Hence, tretinoin, adapalene, and tazarotene, which target RARs, are used in acne, psoriasis, and photoaging (disorders of differentiation and proliferation), whereas bexarotene and alitretinoin, which target RXRs, are used in mycosis fungoides and Kaposi sarcoma (to induce apoptosis of malignant cells).
Retinoid Toxicity. Acute retinoid toxicity is similar to vitamin A intoxication. Side effects of systemic retinoids include dry skin, nosebleeds from dry mucous membranes, conjunctivitis, reduced night vision, hair loss, alterations in serum lipids and transaminases, hypothyroidism, inflammatory bowel disease flare, musculoskeletal pain, pseudotumor cerebri, and mood alterations. RAR-selective retinoids are more associated with mucocutaneous and musculoskeletal symptoms, whereas RXR-selective retinoids induce more physiochemical changes. Because all oral retinoids are potent teratogens, they should be used carefully in females of childbearing potential and not in pregnant patients. Suicide or suicide attempts have been associated with the use of isotretinoin.
TOPICAL RETINOIDS
Through incompletely understood mechanisms, topical retinoids correct abnormal follicular keratinization, reduce P. acnes counts, and reduce inflammation, thereby making them the cornerstone of acne therapy. Topical retinoids are first-line agents for non-inflammatory (comedonal) acne and often are combined with other agents in the management of inflammatory acne.
Fine wrinkles and dyspigmentation, 2 important features of photoaging, also are improved with topical retinoids. Within the dermis, this is believed to result from inhibition of activator protein-1 (AP-1) that normally activates synthesis of matrix metalloproteinases in response to UV irradiation. In the epidermis, retinoids induce epidermal hyperplasia in atrophic skin and reduce keratinocyte atypia.
Toxicity and Monitoring. Adverse effects of all topical retinoids include erythema, desquamation, burning, and stinging (see relative irritancy in Table 65–4). These effects often decrease with time and are lessened by concomitant use of emollients. Patients also may experience photosensitivity reactions (enhanced reactivity to UV radiation) and have a significant risk for severe sunburn. Although there is little systemic absorption of topical retinoids and no alteration in plasma vitamin A levels with their use, it is recommended use of topical retinoids should be avoided during pregnancy.
TRETINOIN
Topical tretinoin (all-trans-retinoic acid) is photolabile and should be applied once nightly for acne and photoaging. Benzoyl peroxide also inactivates tretinoin and should not be applied simultaneously. Formulations with copolymer microspheres (RETIN-A MICRO) or prepolyolprepolymer-2 (AVITA) that gradually release tretinoin to decrease irritancy are available.
ADAPALENE
Adapalene (DIFFERIN) has similar efficacy to tretinoin, but unlike tretinoin, it is stable in sunlight, stable in the presence of benzoyl peroxide, and tends to be less irritating at the 0.1% concentration.
TAZAROTENE
Tazarotene (TAZORAC, AVAGE) is approved for the treatment of psoriasis, photoaging, and acne vulgaris. Tazarotene gel, applied once daily, may be used as monotherapy or in combination with other medications, such as topical corticosteroids, for the treatment of localized plaque psoriasis. Topical corticosteroids improve the efficacy of therapy and reduce the side effects of burning, itching, and skin irritation that are commonly associated with tazarotene.
ALITRETINOIN
Alitretinoin (PANRETIN) is a retinoid that binds all types of retinoid receptors and is applied 2 to 4 times daily to cutaneous lesions of Kaposi sarcoma. Alitretinoin should not be applied concurrently with insect repellants containing diethyltoluamide (DEET, N, N-diethyl-m-toluamide) because it may increase DEET absorption.
BEXAROTENE
Topical bexarotene (TARGRETIN) is approved for early-stage (IA and IB) cutaneous T-cell lymphoma. Its application is titrated up from every other day to 2-4 times daily over several weeks to improve patient tolerance. Patients using bexarotene should avoid products containing DEET due to an increased risk for DEET toxicity.
SYSTEMIC RETINOIDS
Systemic retinoids (Table 65–5) are approved for the treatment of acne, psoriasis, and cutaneous T-cell lymphoma.
Therapeutic Use; Contraindications. Off-label uses include ichthyosis, Darier disease, pityriasis rubra pilaris, rosacea, hidradenitis suppurativa, chemoprevention of malignancy, lichen sclerosus, subacute lupus erythematosus, and discoid lupus erythematosus. Relative contraindications include leukopenia, alcoholism, hyperlipidemia, hypercholesterolemia, hypothyroidism, and significant hepatic or renal disease.
Toxicity and Monitoring. Acute toxicities may include mucocutaneous or laboratory abnormalities; bony changes may occur after chronic use at high doses. Mucocutaneous side effects may include cheilitis, xerosis, blepharoconjunctivitis, cutaneous photosensitivity, photophobia, myalgia, arthralgia, headaches, alopecia, nail fragility, and increased susceptibility to staphylococcal infections. Some patients develop a “retinoid dermatitis” characterized by erythema, pruritus, and scaling. Very rarely, patients may develop pseudotumor cerebri, especially when systemic retinoids are combined with tetracyclines. There are reports that chronic administration at higher doses can cause diffuse idiopathic skeletal hyperostosis (DISH) syndrome, premature epiphyseal closure, and other skeletal abnormalities.
Systemic retinoids are highly teratogenic. There is no safe dose during pregnancy. Although the risk appears to be minimal, men should avoid retinoid therapy when actively trying to father children. Prescribing of isotretinoin in the U.S. is restricted via the risk-mitigation iPLEDGE system. Serum lipid elevation is the most common laboratory abnormality. Less common laboratory abnormalities include elevated transaminases, decreased thyroid hormone, and leukopenia. A baseline evaluation of serum lipids, serum transaminases, and complete blood count (CBC) and a pregnancy test should be obtained prior to starting any systemic retinoids. Laboratory values should be checked monthly for the first 3-6 months and once every 3 months thereafter.
ISOTRETINOIN
Isotretinoin (ACCUTANE, others) is approved for the treatment of recalcitrant and nodular acne vulgaris. The drug has remarkable efficacy in severe acne and may induce prolonged remissions after a single course of therapy. Clinical effects generally are noted within 1-3 months of starting therapy. Approximately one-third of patients will relapse, usually within 3 years of stopping therapy. Although most relapses are mild and respond to conventional management with topical and systemic anti-acne agents, some may require a second course of isotretinoin. There are several reports of patients developing signs of depression while on isotretinoin. Current guidelines recommend monthly monitoring of all patients on isotretinoin for signs of depression.
ACITRETIN
Acitretin (SORIATANE, SORIATANECK) is approved for use in the cutaneous manifestations of psoriasis. Full clinical benefit occurs at 3-4 months. Acitretin has a t1/2 of ~50 h, however, when combined with alcohol, acitretin is esterified in vivo to produce etretinate, which has a t1/2 of >3 months. Thus, female patients of childbearing age should avoid pregnancy for 3 years after receiving acitretin to avoid retinoid-induced embryopathy.
BEXAROTENE
Bexarotene (TARGRETIN) is a retinoid that selectively binds RXRs. Oral and topical formulations of bexarotene are approved for use in patients with cutaneous T-cell lymphoma. Studies suggest that bexarotene induces apoptosis of malignant cells. Because it is metabolized by CYP3A4, inhibitors of CYP3A4 (e.g., imidazole antifungals, macrolide antibiotics) will increase and inducers of CYP3A4 (e.g., rifamycins, carbamazepine, dexamethasone, efavirenz, phenobarbital) will decrease plasma levels of bexarotene. Side effects are more common than with other retinoids, with an increased incidence of significant lipid abnormalities and hypothyroidism secondary to a reversible RXR-mediated suppression of TSH gene expression, pancreatitis, leukopenia, and GI symptoms. Thyroid function should be measured before initiating therapy and periodically thereafter.
VITAMIN ANALOGS
CALCIPOTRIENE. Calcipotriene (DOVONEX, others) is a topical vitamin D analog that is used in the treatment of psoriasis.
Mechanism of Action. Calcipotriene exerts its effects through the vitamin D receptor (VDR) (see Chapter 44). Upon binding the VDR, the drug-receptor complex associates with the RXR-α and binds to vitamin D response elements on DNA, increasing expression of genes that modulate epidermal differentiation and inflammation lead to improvement in psoriatic plaques.
Therapeutic Use. Calcipotriene is applied twice daily to plaque psoriasis on the body, often in combination with topical corticosteroids. Hypercalcemia and hypercalciuria may develop when the cumulative weekly dose exceeds the recommended 100 g/week limit and resolves within days of discontinuation of calcipotriene. Calcipotriene also causes perilesional irritation and mild photosensitivity.
β-Carotene. β-Carotene (see Figure 64–6) is a precursor of vitamin A that is in green and yellow vegetables. No β-carotene products are currently FDA-approved. Dietary supplementation with β-carotene is used in dermatology to reduce skin photosensitivity in patients with erythropoietic protoporphyria. The mechanism of action is not established but may involve an antioxidant effect that decreases the production of free radicals or singlet oxygen. However, a recent meta-analysis concluded that β-carotene, vitamin A, and vitamin E given singly or combined with other antioxidant supplements actually increase mortality. FDA’s Maximum Recommended Therapeutic Dose (MRTD) for β-carotene is 0.05 mg/kg/day.
PHOTOCHEMOTHERAPY
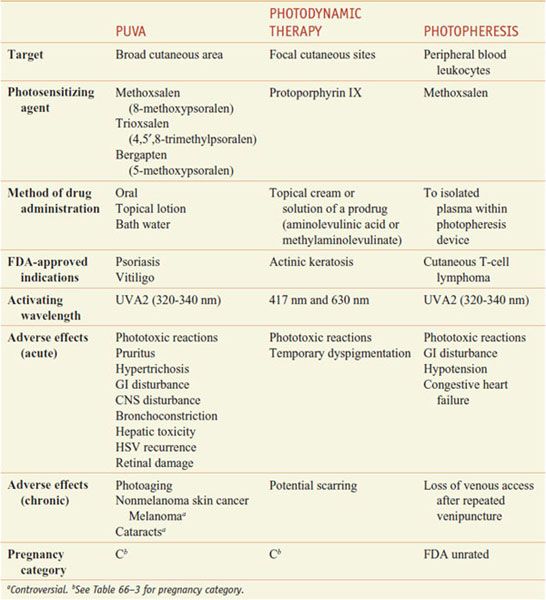
Phototherapy and photochemotherapy are treatment methods in which UV or visible radiation is used to induce a therapeutic response either alone (phototherapy) or in the presence of an exogenous photosensitizing drug (photochemotherapy), such as a psoralen (furocoumarin) derivative that absorbs UV energy and becomes reactive (Table 65–6). Patients treated with these modalities should be monitored for concomitant use of other potential photosensitizing medications, such as phenothiazines, thiazides, sulfonamides, NSAIDs, sulfonylureas, tetracyclines, and benzodiazepines.
Table 65–6
Photochemotherapy Methods
Dermatologists subdivide the UV region into UVB (290-320 nm), UVA2 (320-340 nm), UVA1 (340-400 nm), and UVC (100-290 nm) radiation. UVC radiation is almost completely absorbed by stratospheric ozone. UVB is the most erythrogenic and melanogenic type of radiation. It is the major action spectrum for sunburn, tanning, skin cancer, and photoaging. UVA radiation is only a thousandth as erythrogenic as UVB but penetrates more deeply into the skin and contributes substantially to photoaging and photosensitivity diseases.
PUVA: PSORALENS AND UVA. Orally administered methoxsalen followed by UVA (PUVA) is FDA-approved for the treatment of vitiligo and psoriasis.
Pharmacokinetics. Dissolved psoralens (e.g., OXSORALEN ULTRA) are absorbed rapidly after oral administration, whereas crystallized forms (e.g., 8-MOP) are absorbed slowly and incompletely. Fatty foods also reduce absorption. There is a significant, but saturable, first-pass elimination in the liver. For these reasons, peak photosensitivity varies significantly between individuals but typically is maximal 1-2 h after ingestion. Methoxsalen has a serum t1/2 of~1 h, but the skin remains sensitive to light for 8-12 h.
Mechanism of Action. The action spectrum for oral PUVA is between 320 and 340 nm. Two distinct photoreactions take place. Type I reactions involve the oxygen-independent photoaddition of psoralens to pyrimidine bases in DNA. Type II reactions are oxygen dependent and involve the transfer of energy to molecular oxygen, creating reactive oxygen species. Through incompletely understood mechanisms, these phototoxic reactions stimulate melanocytes and induce antiproliferative, immunosuppressive, and anti-inflammatory effects.
Therapeutic Uses. Methoxsalen is supplied in soft gelatin capsules (OXSORALEN-ULTRA) and hard gelatin capsules (8-MOP) for oral use. The dose is 10-70 mg, depending on weight (0.4-0.6 mg/kg), taken ~2 h before UVA exposure. A lotion containing 1% methoxsalen (OXSORALEN) is available for topical application for vitiligo and can be diluted for use in bath water to minimize systemic absorption. An extracorporeal solution (UVADEX
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree