Gel
Cream
Diltiazem hydrochloride
2 g
2 g
Hydroxyethylcellulose gel DABa
98 g
–
Cream base DACb
–
98 g
Total
100 g
100 g
12.1.2 Adapting Licensed Products
In general adapting licensed preparations is problematic for several reasons:
Scientific evidence of therapeutic rationality, activity and safety of the adapted product is lacking.
Additives can change biopharmaceutical properties of the licensed product.
The combination of two licensed products may decrease concentrations of the active substances too much.
The duration of the treatment with one or the other active substance may be typically different, causing the patient to be treated inadequate, either too short or too long. For example the combination of antibiotics and corticosteroids can result in bacterial resistance if the antibiotic is applied too long. Upon application of the combination of corticosteroids and antimycotics the patient may be treated too long with the corticosteroid or the treatment with the antimycotic is insufficient.
The qualitative and quantitative formula of licensed pharmaceutical preparations is often not known. Adding new active substances or excipients may result in physical and chemical incompatibilities.
12.1.3 Recommendations
The following general recommendations may be helpful for handling prescriptions of cutaneous preparations:
If suitable licensed medicinal products are available, they should be preferred.
If a licensed product has to be adapted, the pharmacist should suggest a standard pharmacy preparation with a comparable base and chemical form of the active substance. For example the type of emulsion has to be the same or an anhydrous formulation such as a hydrophilic or hydrophobic ointment should be replaced with a similar anhydrous preparation.
Is combination or adaptation of licensed products aimed at a better adherence, it is to be preferred that physician and pharmacist give clear advice about the administration of the original products or start from a standard pharmacy formulation. For the instruction of patients where, how, how often and how long to apply cutaneous preparations, a special form with an outline figure may be helpful, see Fig. 2.5.
Standard formulations in national formularies are to be preferred. The addition of another active substance to standard formulations is possible, but the number of substances has to be limited and incompatibilities must be excluded.
In case of non-standardised preparations the pharmacist should professionally improve the formulation if necessary, for instance by the addition of buffers and choosing a similar but compatible base.
To ease the communication between pharmacist and physician some defined agreements are useful. The pharmacist has to identify the problems in the prescription and should name alternatives to the formulation if needed. The concept of the treatment has to be considered. The following measures can be agreed upon beforehand:
Obsolete active substances or excipients should not be processed.
Preservatives are added if microbial growth is expected. If a formulation should be prepared without a preservative, the physician has to note that on the prescription.
Overdosing for therapeutic reasons has to be noted on the prescription, for example with an exclamation mark.
The declaration of the chemical form of steroids for dermal use has to be clear. Some of the steroids have no effect on the skin, for example triamcinolone or betamethasone, but the esters do.
Prescribing extemporaneous cutaneous preparations is a privilege that many physicians appreciate as well as some patients. This may cause a conflict of interest because the physician wants to prescribe a tailor made preparation, but the pharmacist may not be able to formulate and supply a stable and effective product, for example because of incompatibilities, toxicological or quality concerns. In order to agree on which preparations can be prescribed and supplied it has been proven useful if pharmacists and physicians have access to a standardised and rationalised assortment of cutaneous pharmacy preparations, based on literature and multidisciplinary guidelines. Examples are “Dermatica op Recept” in the Netherlands [2] or “Standardisierte Rezepturen – Formelsammlung für Ärzte” in Germany [3]. If the physician needs a non-standardised preparation a full prescription assessment (see Sect. 2.2) should be performed.
12.2 Definitions
12.2.1 Classification of the European Pharmacopoeia
In the eighth edition of the European Pharmacopoeia cutaneous preparations are classified as liquid preparations for cutaneous application, semisolid preparations for cutaneous application and powders for cutaneous application [4].
Liquid preparations for cutaneous application are described as preparations with variable viscosity intended for local or transdermal application. This category comprises solutions, emulsions and suspensions for dermal use containing one or more active substances in a suitable base. As examples for liquid preparations shampoos and cutaneous foams are defined in Ph. Eur.
Semisolid preparations for cutaneous application are subdivided in ointments, creams, gels, pastes, poultices (wet dressings), medicated plasters or patches and cutaneous patches.
Ointments are cutaneous preparations consisting of one phase, hydrophilic or hydrophobic, in which a solid phase may be dispersed. They are subdivided in hydrophobic ointments, water-emulsifying ointments and hydrophilic ointments.
Hydrophobic ointments are ointments that can absorb only small quantities of water. The base of water emulsifying ointments is similar to the base of the hydrophobic ointments but also contains one or a few emulsifying agents, so they can absorb larger quantities of water than hydrophobic ointments and form water-in-oil (w/o) or oil-in- water (o/w) emulsions, depending on the type of the emulsifier. Hydrophilic ointments are ointments which consist of only a hydrophilic phase. They are miscible with water.
Creams are described as preparations comprising a lipophilic and an aqueous phase. Creams with the aqueous phase as continuous phase are classified as hydrophilic. Lipophilic creams are creams with a lipophilic continuous phase.
Gels are defined as fluids that form a gel because of the presence of a suitable gelling agent. Gels are subdivided in hydrophilic and lipophilic gels.
Pastes are semisolid preparations containing a large amount of solids, dispersed in the base.
Poultices (wet dressings) are hydrophilic heat-retentive bases in which one or more active ingredients, solids or fluids, are dispersed. Generally, they are heated before use and applied on the skin in a thick layer.
Medicated plasters are flexible preparations with one or more active substances that should be slowly absorbed, or they have protective or keratolytic properties.
Cutaneous patches are intended for local effects.
Powders for cutaneous application consist of solid, loose, dry particles with a varying fineness. They may contain one or more active substances and excipients.
12.2.2 Classification in Practice
Official definitions for the different types of cutaneous preparations are given in the European Pharmacopoeia (Ph. Eur.). But in the physicians practice, in licensed preparations and in formularies various terminologies can be found. For example a physician usually associates cream with a hydrophilic base and ointment with a lipophilic one. In the German Pharmacopoeia the Hydrophilic ointment with water (Unguentum emulsificans aquosum) is not an ointment as the Latin term Unguentum suggests. It is on the contrary a hydrophilic cream by Ph. Eur. definition. Emulsions in the sense of liquid preparations with a high amount of water are often named lotions, liniments or milks. As the pharmacist has a lot of different bases for cutaneous preparations it makes sense to communicate the therapeutic goal, the skin conditions and the area of application with the physician to find the right formulation. Knowledge of the characteristics and properties of cutaneous preparations helps to make an appropriate choice. Table 12.2 shows the official terminology in Ph. Eur. EDQM standard terms [5], synonyms used in physicians’ practice and European formularies and formulations to be found in this chapter as references for each type of preparation.
Table 12.2
Definition of cutaneous preparations
Ph. Eur. | Standard terms | Synonyms | Examples |
|---|---|---|---|
Powders for cutaneous application | |||
Cutaneous powder | Base for cutaneous powder (Table 12.18) | ||
Liquid preparations for cutaneous application | |||
Solution | Cutaneous solution | ||
Suspension | Cutaneous suspension | Mixture, lotion | Zinc oxide cutaneous suspension (Table 12.21) |
Emulsion | Cutaneous emulsion | Liniment, milk, lotion | Cetomacrogol cutaneous emulsion (Table 12.24) |
Semisolid preparations for cutaneous application | |||
Lipophilic gel | Gel | Oleogel | Hydrophobic base gel DAC [43] |
Hydrophilic gel | Mucilago, hydrogel | Hydroxyethylcellulose gel DAB (Table 12.35) | |
Lipophilic cream | Cream | W/o cream | |
Hydrophilic cream | O/w cream | ||
Hydrophobic ointment | Ointment | Coal tar soft paraffin ointment (Table 12.25) | |
Water-emulsifying ointment | |||
Hydrophilic ointment | Macrogol ointment DAC (Table 12.29) | ||
Paste | Cutaneous paste |
12.3 Biopharmaceutics
In cutaneous preparations the base as such has an influence on the therapeutic effectiveness. It also very much influences the therapeutic effect of the active substance by influencing its release and penetration into the skin. To understand how penetration of active substances and the therapeutic effect are related to the formulation, it is necessary to have knowledge on the anatomy of the skin and skin biopharmaceutics. Release and penetration into the skin is generally described in Sect. 16.2.5. As the therapeutic effectiveness of cutaneous preparations also depends on the frequency of application and the amount to be dispensed, these aspects are also dealt with.
12.3.1 Anatomy of the Skin
From the outside to the inside the skin consists of the following layers (see Fig. 12.1):
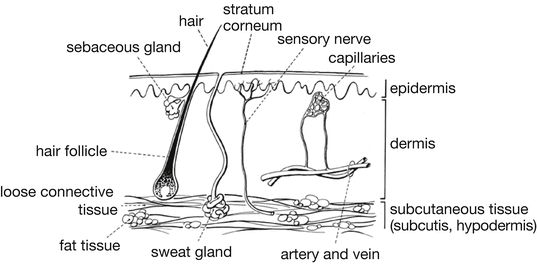

Fig. 12.1
Anatomy of the skin. Source: Recepteerkunde 2009, © KNMP
The epidermis, which has mainly a protecting function
The dermis (corium), which has a supplying and stabilising function
The subcutis, a layer which has an insulating and protecting function and works as an energy storage
The epidermis consists of a multi-layered epithelium that varies in thickness from 0.03 mm on the eyelids up to 2.0–4.0 mm on the palms of the hands and soles of the feet. The cells are gradually and continuously renewed by being moved from the inside to the outside of the skin. During this outward migration the cells change. They keratinise and disintegrate and become denucleated forming a firm cornified layer. From the outside to the inside the epidermis consists of five layers: the stratum corneum, the stratum lucidum, the stratum granulosum, the stratum spinosum and the stratum germinativum. In the stratum germinativum, the cell division takes place. The interior four layers are called the living epidermis.
The outer layer of the epidermis, the stratum corneum, consists of dead, mainly keratine containing cells that are continuously scaled off. It is considered as a membrane, built from a dense lipid protein matrix. The function of the stratum corneum is to form a barrier to protect underlying tissue from infection, dehydration, chemicals, radiation and mechanical stress. During the process of division, migration and death of the epidermis cells the percentage of water decreases from approximately 70 % in the stratum germinativum to 10–20 % in the stratum corneum. However, the stratum corneum can absorb more water (up to 50–75 %), for example under occlusion or while bathing. Hydrated the stratum corneum has a better permeability and the protecting function is decreased. The pH is 7.1–7.3 in the stratum germinativum and 5.4–5.9 in the stratum corneum.
The dermis has a thickness of about 3–5 mm. In contrast to the epidermis it contains blood vessels and nerves. The dermis is divided into two layers. The outer layer, the papillary region contains a network of capillaries and has a supplying function for the epidermis. The inner layer, the reticular region lies under the papillary region and is usually much thicker. Because of the high concentration of collagenous and elastic fibers imbedded in dense connective tissue, it gives the dermis mechanical strength and elasticity.
The subcutaneous tissue mainly contains fat tissue. It has an insulating and buffering function.
Hairs stand loose in hair follicles. Each root of a hair is connected with a sebaceous gland that is secreting sebum into the follicle. Sebum consists of short chain fatty acids, waxes and emulsifiers, such as cholesterol. Sweat glands are coiled tubes that range from the dermis and subcutis into the surface of the skin. The fluid produced by the glands, the sweat, is a hypotonic salt solution with a pH of approximately 5. Beside salts, it contains small quantities of other substances such as lactic acid and urea. The most important function of sweat glands is temperature regulation by loss of water vapour. The mixture of sebum and secreted products of sweat glands and the loose dead cells of the stratum corneum form a greasy layer, which covers the skin. This lipid film can absorb water because of the presence of emulsifying agents. However it remains water resistant and protects the skin against dehydration. The short chain fatty acids originating from sebum and sweat components and the low pH are responsible for its protecting effect against pathogenic micro-organisms.
12.3.2 Release and Penetration
See Sect. 16.5.1 at first. The rate and extent of penetration of the active substance into the skin mainly depends on the partition of the active substance in the base and the stratum corneum. Other factors such as skin conditions, the area and method of application play a role as well.
Hydrocortisone and the acetate and butyrate esters are often used for cutaneous application. In the skin the esters hydrolyse rapidly and the active substance hydrocortisone is formed. Usually the concentration of hydrocortisone acetate in creams is 10 mg/g. The usual concentration of hydrocortisone butyrate is 1 mg/g, 10 times lower than the hydrocortisone acetate ester.
Glucocorticosteroids for external use are classified by their potency. Two systems of classification exist that must not be mixed up. One is depicted in the WHO Model prescribing information [6]: The steroids for external use are assorted there in four groups with seven classes from class I (ultra high potency) to VII (low potency). The more common and well known system of Niedner [7] has only four categories from class I (low potency) to class IV (very high potency). Criteria for this classification system are the local effect in the vasoconstriction test, the anti-inflammatory and the anti-proliferative effectiveness.
Preparations with hydrocortisone acetate are classified in activity class I, preparations with hydrocortisone butyrate in class II. Although the active substance in both preparations is the same, the therapeutic effectiveness of the butyrate ester is stronger. This is the consequence of the higher lipophilicity of the butyrate ester that leads to better skin penetration.
If substances are in a dissolved state near the skin surface, they will penetrate into the skin rapidly after application. Dissolved molecules in the preparation firstly have to diffuse towards the skin surface. If the rate of diffusion through the base is low, the penetration will be impeded. The rate of diffusion through the base depends on the properties of the active substance and the base.
If the active substance is suspended in the base, it must dissolve first. This process depends on its solubility in the base. A small particle size increases the dissolution rate.
The properties of the active substance influence skin penetration as well. Generally lipophilic molecules penetrate into the skin more rapidly than hydrophilic substances. Additionally, lipophilic substances are released more rapidly from aqueous than from lipophilic bases because of their higher affinity to the stratum corneum.
The base of a cutaneous preparation also influences the rate and extent of absorption. The base can be simple, such as an aqueous solution, but can also be very complex such as an emulsion. The base can alter the skin conditions, for example by hydration. As a result, the penetration of the active substance into the skin may be modified.
Ultimately, the release and penetration of active substances from cutaneous preparations is difficult to predict.
Lidocaine is applied on the skin in its non-ionic form because this form penetrates best. To anaesthesise mucous membranes the hydrochloride salt of lidocaine is more suitable because the charged form dissolves better in the mucous membranes than the base.
Cutaneous preparations for the skin should contain the base as such or the base has to be formed from lidocaine hydrochloride. Three concepts may be followed:
1.
Lidocaine hydrochloride is dissolved in an aqueous gel. As only non-dissociated molecules can penetrate into the skin, and lidocaine is slightly soluble in water, the pH is adjusted to 6.5. At this pH only a small amount of lidocaine (pKa = 7.9) is in the lipophilic form, but dissolved anyway.
2.
Lidocaine is dissolved in a lipophilic phase by warming. An aqueous phase is added last.
3.
Lidocaine is added by deliquescing lidocaine crystals and levomenthol together. The eutectic mixture is than emulsified in an aqueous gel. The biopharmaceutic advantage of this principle is that a molecular dispersion of the lipophilic lidocaine base in a hydrophilic preparation is formulated.
Penetration into the skin can be enhanced by penetration enhancers. These excipients diffuse into the stratum corneum and interact with components of this layer. The barrier function of the skin decreases. The effect of penetration enhancers is based on two mechanisms. The penetration enhancer can change the structure of the stratum corneum or the solubility of the active substance in the skin. Penetration enhancers should not damage the underlying skin layers and should not be toxic or allergenic. Moreover, the effect must be reversible. Because of the different properties and mechanisms of action of penetration enhancers it is difficult to predict which enhancer will be most effective for the penetration of a specific active substance. Substances such as dimethyl sulfoxide (DMSO), salicylic acid, urea, propylene glycol, ethanol, isopropyl alcohol and many acids can act as penetration enhancers.
To be effective, a locally applied corticosteroid must penetrate into the skin. The rate and extent of penetration is determined for a large extent by the formulation of the base. By adding penetration enhancers such as salicyl acid or urea the penetration of the active substance is enhanced. In this way a class I or II corticosteroid containing preparation becomes more effective.
12.3.3 Choice of the Base
The base of cutaneous preparations does not only influence the release of the active substance, it can contribute to the therapeutic effectiveness of the preparation itself. In some cases the base alone may sufficiently influence healing, by which the addition of an active substance is unnecessary. In such cases the physical properties of the base are utilised, such as: cooling; dehydration or protection by indifferent solids; prevention of dehydration by hydrophobic components. The correct choice of the base is very important for wet and dry skin disorders. If the skin is neither dry nor wet the choice of the base is much less important than the active substance. The classification of a number of bases appropriate for several skin conditions has been summarised in Table 12.3 for a gradual transition from wet to dry skin and for specific skin conditions.
Table 12.3
Basic preparations for different skin conditions and skin types
Skin | Form | Effect | Base |
|---|---|---|---|
Wet skin | Water or wet dressing | Drying, astringent | Water, physiological saline solution, black tea as wet dressing |
↓ | |||
↓ | |||
↓ | Cutaneous suspensions (commonly with zinc oxide) | Drying | Zinc oxide cutaneous suspension (Table 12.21) |
Pastes (commonly with zinc oxide) | Drying | Zinc oxide aqueous paste (Table 12.41) | |
Hydrophilic cream | Neutral | ||
Dry skin | Lipophilic cream | Hydrating | Hydrophobic cream base DAC (Table 12.32) |
Strong keratotic disorders | Ointment | Strongly hydrating, occluding | Emulsifying hydrophobic base gel DAC (Table 12.26), White soft paraffin |
Greasy (oily) skin | Hydrophilic gel | Hydroxyethylcellulose Gel DAB (Table 12.35) | |
Solution | Blends of ethanol/isopropyl alcohol, propylene glycol and water | ||
Scalp (seborrheic) | Hydrophilic gel | Hydroxyethylcellulose Gel DAB (Table 12.35) | |
Solution | Blends of ethanol/isopropyl alcohol, propylene glycol and water | ||
Scalp (dry) | Solution (washable, oily) | Vegetable oils, octyldodecanol, triglycerides medium-chain blended with surfactants (for example macrogol lauryl ether) | |
Open wounds | Solution (sterile) | Physiological saline solution | |
Hydrophilic gel (sterile) | Isotonic carbomer gels |
Usually the base of a cutaneous preparation is prescribed by the physician. But sometimes the pharmacist is free to choose it on his own authority. Both, physician and pharmacist, have to take the following factors into consideration:
The base should not irritate or sensitise.
The base should not adversely influence the active substances.
The base has to be cosmetically acceptable, preferably not shiny and not sticky after spreading on the skin.
The choice of the base depends on the skin conditions (whether the disease is in an acute or chronic state) and the skin type.
12.3.4 Base and Different Skin Disorders
12.3.4.1 Acute Skin Disorders
Acute exsudative skin disorders are usually treated with wet packs first, for example with black tea because of the astringent effect of the tannin content. Dressings wetted with physiological saline solutions or water are used as well. As a result of evaporation of water the dressings will absorb fluid and purulence from the skin. The dressings have to be changed a few times a day. They should not be wrapped in plastic because that prevents water from evaporating and the dressings will not be effective. The treatment with wet dressings results in cooling and drying of the skin.
Not recommended for the treatment of acute exudative skin disorders are:
Hydrophilic suspensions, because the powder that remains on the skin after drying can irritate and damage it
Powders for cutaneous application for the same reason, except powders consisting of absorbable sterile lactose
For the acute state of the disorder, if without strong exudation, hydrophilic suspensions are appropriate. They typically contain a relatively high amount of water and solid substances. Solid substances act as a drying agent on the skin because of their water absorbing property. The combination of a solid substance and water increases the evaporation surface area of water. This increases the cooling effect. Examples for such preparations are zinc oxide cutaneous suspensions.
When the skin has become dryer and is changing to a subacute state it can further be treated with a hydrophilic cream.
12.3.4.2 Normal Skin
For patients with normal skin conditions and also for patients with a subacute dermatosis hydrophilic creams are frequently used. If a preparation with more fat is needed for a slightly drier skin, hydrophilic creams with a higher amount of lipophilic components are applied. For an example a cream with added soft paraffin (Table 12.4).
Cetomacrogol emulsifying wax (BP) | 15 g |
Paraffin, liquid | 12.5 g |
Paraffin, white soft | 22.5 g |
Propylene glycol | 10 g |
Water, purified | 40 g |
Total | 100 g |
A slightly dry skin may be treated with an extra fat containing hydrophilic cream. Patients with a very dry skin use cutaneous preparations with a high amount of lipophilic components. Lipophilic creams are suitable for the treatment of the chronic state of atopic eczema. Hydrophobic ointments or saturated hydrocarbons are not indicated because they have an occlusive effect, which causes heat accumulation and enhances itching.
12.3.4.3 Strong Keratotic Disorders
A strong keratotic skin is treated with hydrophobic ointments. Hydrophobic anhydrous bases cover the skin and prevent evaporation of water, the skin becomes hydrated. Examples for such disorders are ichthyosis or psoriasis.
12.3.4.4 Greasy (Oily) Skin
For a seborrheic skin hydrophilic bases have to be chosen: hydrophilic gels, emulsions or creams. Especially for acne treatment bases without or with only a small percentage of lipophilic components are indicated.
12.3.4.5 Itching Skin Disorders
Hydrophilic suspensions (liniments, lotions) are suitable for application on large itching areas if the skin is not damaged. The evaporation of the liquid components will cool the skin and reduce the itch.
12.3.4.6 Scalp
For the treatment of the scalp hydrophilic solutions and hydrogels, liquid emulsions and washable liquid oils are used. Examples are olive, almond or refined castor oil, as well as fatty alcohols such as octyldodecanol, all in combination with a surfactant, for instance macrogol lauryl ether 4. The decision for one or the other base depends on the skin conditions. A seborrheic scaling scalp is often treated with hydrophilic preparations, a dry and scaling one is treated with liquid oils.
12.3.4.7 Piles
Hydrophilic creams with a high amount of lipophilic components are appropriate for the treatment of haemorrhoids. These creams cover the painful, damaged mucous membrane. Soft Paraffin Cetomacrogol cream FNA or Cream base DAC (Tables 12.4 and 12.6) are two examples for suitable bases. Thinner creams may flow off easily. A more lipophilic base is not desirable because of the chance on maceration: the weakening of the skin by long time exposure to moisture.
12.3.4.8 Open Wounds
In modern wound management aqueous solutions (irrigations) and hydrogels are mentioned to be applied on deep and chronic wounds. Other preparations such as creams ointments and powders are described as well but have some disadvantages. Components of these preparations may not be removed from the wound easily and lipophilic compounds can hinder secretion. Cutaneous preparations with zinc oxide should not be used on open wounds because they dry out the edges of the wound. The consequence is delayed wound healing. Talc can cause the formation of granulomas and should therefore be avoided as well. As a typical powder base for wound powders absorbable sterile lactose is often used. In general cutaneous preparations that are applied to large damaged areas of the skin (wounds) must be sterile, because the natural barrier of the skin against micro-organisms is lacking. The specific formulations are described in Sect. 12.7.17.
12.3.5 Method of Application and Dosing
12.3.5.1 Method of Application
In general hydrophilic creams (o/w-creams) and emulsions are used without a bandage. Whether to cover the application site after application of lipophilic creams (w/o-creams) and ointments depends on the skin conditions. To soften a skin area with a strong keratinisation covering the lipophilic preparation with a bandage is effective. Fatty pastes and pastes with a cooling effect can be put on lint first. Thereafter the application site is dressed with a bandage.
12.3.5.2 Quantity to Be Applied
If the cutaneous preparation is going to be applied because of its physical properties, such as the prevention of dehydration, ample application is desirable. If the preparation is mainly acting by its active substance, the amount to be applied is expressed in fingertip units to prevent underdosing when the patient is told “to apply thin”, as well as overdosing [9].
The FTU reflects a stripe of cream or ointment with a length from the tip of the index finger of an adult to its first crease. One FTU is approximately 0.5 g of cream or ointment. This is sufficient to cover 300 cm2 of skin. Depending on the body part to be treated more FTU’s can be applied (see Table 12.5).
Table 12.5
FTU dosing
Head and neck | Arm and hand | Leg and foot | Torso (front side) | Back and bottom | Whole body | |
|---|---|---|---|---|---|---|
Age | Number of FTU (fingertip units) | |||||
3–12 months | 1 | 1 | 1½ | 1 | 1½ | 40 |
1–2 years | 1½ | 1½ | 2 | 2 | 3 | 24 |
3–5 years | 1½ | 2 | 3 | 3 | 3½ | 18 |
6–10 years | 2 | 2½ | 4½ | 3½ | 5 | 14 |
adults | 2½ | 4a | 8b | 7 | 7 | 8 |
Another approach is that for hydrophilic creams the amount should be limited to as much as the skin can absorb. Lipophilic creams and hydrophobic ointments should be applied in a thin layer until the skin feels slightly fatty.
12.3.5.3 Application Frequency
Neutral bases should be applied at least twice a day, but may be applied as often as desired. Cutaneous application of corticosteroids results in the formation of a depot of the active substance in the stratum corneum. As a consequence the frequency of application may be reduced after some time. Application once a day is generally sufficient for effective therapy.
For the treatment with corticosteroids several options of therapy are established:
Interval therapy implies treatment with the corticosteroid containing preparation for some days alternating with the base for some days.
Gradual therapy starts with a stronger corticosteroid in the initial phase for up to 7 days. Then step by step weaker corticosteroids are used.
Proactive therapy: After the symptoms have disappeared, the application of the corticosteroid will be continued with a frequency of two times a week.
12.3.5.4 Duration of Therapy
How long a cutaneous preparation has to be used depends on the disorder and the typical period in which an active substance reaches its therapeutic effect. Usually corticosteroids or antibiotics should be applied for only a few days if applied every day. The treatment with an antimycotic may take a longer time. In general the physician should see the patient regularly to assess the effectiveness of the therapy. The preparation should therefore be prescribed in a limited amount appropriate to the treatment scheme.
12.3.6 Occlusive and Transdermal Preparations
Cutaneous preparations are inefficient formulations since only small amounts of the applied active substance penetrate the skin and reach the site of action. The first attempts to understand the mechanism of skin permeation and formulation effects of cutaneous preparations were described in 1960 [10]. Since then more research has been performed on rational design of dermal formulations. Much research is focused on improved skin penetration [11, 12]. Skin penetration is discussed in detail in Sect. 16.2.5 Dermal and transdermal administration.
Relatively new developments of enhanced skin penetration are cutaneous and transdermal patches. The European Pharmacopoeia distinguishes medical plasters and cutaneous patches which are classified as ‘Semisolid Preparations for Cutaneous Application [4] and transdermal patches which are described in a separate monograph [4].
12.3.6.1 Cutaneous Patches
According to the European Pharmacopoeia ‘cutaneous patches are flexible preparations containing 1 or more active substances. They are intended to be applied to the skin. They are designed to maintain the active substance(s) in close contact with the skin such that these may act locally’. Cutaneous patches consist of an adhesive basis spread as a uniform layer on an appropriate support made of natural or synthetic material. The adhesive basis is not irritant or sensitising to the skin. The adhesive layer is covered by a suitable protective liner, which is removed before applying the patch to the skin.
Skin permeation of the active substance after application of cutaneous patches is enhanced compared to conventional cutaneous preparations because of the occlusive effect of the patch. In this way relatively high concentrations in the skin are obtained. In contrast to transdermal patches plasma concentrations will be low and no systemic side effects are observed.
Examples of cutaneous patches are lidocaine containing patches that sometimes contain a second local anaesthetic. After applying the patch, lidocaine penetrates deep into the skin where it has a local anesthetising effect. Capsaicin containing patches are used in the treatment of peripheral neuropathic pain in non-diabetic adults. Following exposure to the patch, capsaicin penetrates the skin and interacts with the cutaneous transient receptor potential vanilloid 1 receptor (TRPV1) resulting in pain relief.
12.3.6.2 Medicated Patches
The European Pharmacopoeia also defines medicated plasters as semisolid preparations for cutaneous application. According to the European Pharmacopoeia “medicated plasters are flexible preparations containing 1 or more active substances. They are intended to be applied to the skin. They are designed to maintain the active substance(s) in close contact with the skin such that these may be absorbed slowly, or act as protective or keratolytic agents”. 5-Aminolevulinic acid medicated plasters represent this dosage form. They are used in photodynamic/radiation therapy. The plasters are applied to mild to moderate actinic keratosis lesions. Four hours after application the plasters are removed and the lesions are exposed to red light.
12.3.6.3 Transdermal Patches
In contrast to cutaneous patches and medicated plasters, transdermal patches are designed for delivery of an active substance into the systemic circulation and consequently to achieve a systemic effect.
According to the European Pharmacopoeia “transdermal patches are flexible single-dose preparation intended to be applied to the unbroken skin to obtain a systemic delivery over an extended period of time”. Manufacturers design patches in a variety of ways. However, in general patches can be categorised in two main types: the reservoir and the matrix systems. In reservoir systems the active substance may be dissolved or dispersed in a semisolid basis or in a solid polymer matrix, which is separated from the skin by a rate-controlling membrane. Matrix systems contain the active substance in a solid or semisolid matrix, the properties of which control the diffusion pattern to the skin. The matrix system may also be a solution or dispersion of the active substance in the pressure-sensitive adhesive. The releasing surface of the patch is covered by a protective liner to be removed before applying the patch to the skin. The principles of different types of transdermal patches are given in [13]. To improve drug delivery to the systemic circulation transdermal patches often contain excipients to enhance penetration into the skin.
Transdermal patches are an alternative for patients that are not able to take oral medication. Advantages of transdermal patches are that they are easily applied and may prevent more painful and arduous parenteral administration. Moreover, transdermal administration is advantageous for active substances that undergo extensive first-pass metabolism, active substances with narrow therapeutic window or active substances with a short half-life, which cause noncompliance due to frequent dosing.
For transdermal drug transport the active substance should have appropriate physico-chemical and pharmacological properties. Although different requirements are described in literature, in general the molecular weight of the active substance should not exceed about 500 Da, the partition coefficient (log P(octanol/water)) should be between 1.0 and 4.0 and the dose should be low (less than 20 mg/day). Recent research focusses on transdermal delivery of active substances that do not meet these requirements, such as relatively large molecules.
Transdermal patches registered at present are patches containing active substances such as buprenorphine, estradiol, fentanyl, glyceryl trinitrate, nicotine, oxybutynine, rivastigmine, rotigotine and scopolamine. These transdermal patches are used in the treatment of a variety of diseases. They release the active substance during a period of 24 up to 72 h, depending on the type of patch used and the active substance. After application of the patch plasma concentration slowly rises until a steady concentration is reached. During application a depot of the active substance is formed in the skin. After removal of the patch plasma concentrations will gradually but slowly diminish. Because of the absorption of the active substance from the depot in the skin even after the patch has been removed, elimination from the plasma will be slower than anticipated based on i.v. or oral elimination data. Therefore, when switching from transdermal patches to oral medication it should be taken into account that the active substance is still present in the systemic circulation. The oral dosing time and dose should be adapted accordingly.
Well known as transdermal patches are those containing fentanyl. Fentanyl is used in the treatment of severe chronic pain. Fentanyl is rapidly metabolised in the liver resulting in low bioavailability after oral administration. After application of the patch, 90 % of the released amount reaches the systemic circulation. After first application the plasma concentration increases gradually, stabilises after 12–24 h and is stable up to 72 h [14]. Fentanyl transdermal patches often offer an adequate alternative to parenteral opioid administration in patients who are not able to take their medications orally.
12.4 Adverse Effects
Adverse effects of cutaneous preparations may be: undesirable systemic effects, (photo) toxicity, irritation or (photo) allergic responses after sensitisation. Substance monographs [15], package leaflets or SmPCs of licensed products and medicine databases give information about these adverse effects.
Toxic systemic effects have been reported for salicylic acid, resorcinol, lindane or mercury substances. These effects are related to the substance, the amount of preparation and the body surface area to which it is applied, the skin conditions and duration of treatment. Symptoms for systemic intoxications are for example headache, nausea and vomiting, convulsions, fall in blood pressure, kidney damage or metabolic acidosis. Apart from salicylic acid the mentioned substances are no longer used because of these systemic adverse effects and the limited therapeutic significance in cutaneous preparations. Especially infants and toddlers are susceptible for systemic adverse effects because their skin is thinner. Additionally, the relative body surface area in relation to body contents in children is larger than in adults. For salicylic acid in infants and toddlers the only indication is psoriasis. It should be used in low concentrations and on a limited body surface area.
Irritation is induced by active substances that chemically damage cells of the skin. The strength of the effect depends on the concentration of the irritating substance. Irritation is expressed by redness, itching and sometimes erosion of the skin. Decreasing the concentration can prevent irritation. However, the therapeutic effect will be reached later as well. As irritation also depends on the individual sensitivity of the patient´s skin the tolerance differs individually. An example of an irritating active substance is tretinoin. The decrease of the concentration can lead to better tolerance. Some patients have to get used to the irritating effect in order to be able to tolerate later during the treatment higher or more irritating concentrations. Some active substances that are known to be irritating in cutaneous preparations are benzoyl peroxide, dithranol, hydroquinone, iodine, capsaicinoids and salicylic acid.
An allergic reaction is an immunogenic reaction that may be limited to redness of the skin but may also be severe. An allergic reaction occurs if the patient is sensitised against the substance. The risk of getting sensitised via the skin is relatively high. Once a person is sensitive to a substance, he or she will never tolerate the substance anymore. If the allergic reaction is caused by an excipient, the active substance can still be used, but in a base with different excipients. Excipients that are known to be sensitising are methyl parahydroxybenzoate and propyl parahydroxybenzoate, wool alcohols and cetostearyl alcohol. Examples for sensitising active substances are neomycin, tetracain and clioquinol.
A photosensitisation or phototoxic reaction occurs if the skin is exposed to sun light or artificial sun light (e.g. solarium). Examples of substances that are known to cause such reactions are benzoyl peroxide, coal tar, methoxsalen and essential (volatile) oils. Intensive exposition to (artificial) sunlight has to be avoided while and after using these substances. Appropriate clothing and sunscreen are recommended. Methoxsalen and in special cases coal tar are applied for a treatment with ultraviolet light. It is monitored by the physician and the photosensitisation is desired.
Some medicines can cause abnormal reactions to sun light or artificial light equally whether they are intended for oral or dermal administration. The phototoxic reaction may be toxicological or immunological. It is very difficult to distinguish a phototoxic reaction from a photoallergic one. Moreover, a combination of these reactions also occurs. The substance is than phototoxic as well as photoallergic.
12.5 Product Formulation
Cutaneous preparations may consist of a simple or a more complex formulated base in which one or more active substances can be dissolved, dispersed or mixed. Bases often contain several phases: a solid phase, an aqueous, a lipophilic phase and an interphase. In this section the properties, function and excipients of each phase are discussed. Active substances or excipients may make up the solid phase. The aqueous phase may contain the active substance, excipients that improve the microbiological stability or sometimes excipients that improve physical stability of the preparation. The lipophilic phase influences and improves the consistency of the cutaneous preparation. If there is an aqueous and a lipophilic phase in the preparation, there is also an interphase. In cutaneous preparations a number of specific emulsifying agents are applied, which are described. Because of the presence of several phases, the physical and chemical stability and potential incompatibilities have to be considered in the design of the formulation. Last but not least the preservation and packaging which can play a role in the chemical, physical and microbiological stability and shelf life of cutaneous preparations are important issues of this section.
12.5.1 Solid Phase
The solid phase contains solid substances which are dispersed in a liquid phase or a semisolid base. Powders for cutaneous application consist only of a solid phase. The solid substances can be active substances or excipients.
12.5.1.1 Particle Size
The particle size of solid substances of both excipients and active substances is important not just for biopharmaceutical properties (see Sect. 12.3.2) but also for the physical and chemical properties of cutaneous preparations. If the solid is dispersed in a liquid base, the particles must be sufficiently fine to obtain a physically stable suspension (see Sect. 18.4.2). However decrease of particle size may increase the rate of degradation. Additionally processing small particles may lead to agglomerates.
Solid particles should be small enough not to be felt when being applied onto the skin of the patient. The Ph. Eur. doesn’t give detailed guidance for particle size in the chapters for cutaneous dosage forms (see Sect. 12.2) just that it has to be suitable for application. A maximum particle size of 90 μm in powders, hydrophobic and hydrophilic ointments and in creams is generally accepted and therefore recommended.
Many solid raw materials are meant to be used in cutaneous preparations are processed in their micronised state (see Sect. 23.1.8 for definitions) mainly for improving the release of the active substance (see Sect. 12.3.2).
In case of solutions small particles may increase the speed of dissolving.
Tetracycline hydrochloride is in the Netherlands available in two qualities, microcrystalline and ‘ponderosum’ (‘heavy’). Tetracycline hydrochloride ponderosum consists of agglomerates of the microcrystalline substance and has a shelf life of 3 years, has a better flowability and is much less susceptible to degradation than the microcrystalline form. It is mainly used for capsule preparation. Because of the small particles microcrystalline tetracycline hydrochloride is easier to process in cutaneous preparations than the ponderosum quality. The disadvantage of the microcrystalline quality is that during storage epi- and anhydro degradation products may be formed. Only 1 % of these substances are allowed in tetracycline hydrochloride [16]. The degradation is visible as a discolouration to a darker yellow. Generally a cream prepared with the ponderosum quality has a lighter colour than a cream containing the microcrystalline quality.
In practice the more stable ‘ponderosum’ quality can be dispersed in cutaneous preparations anyway by firstly triturating with water in a mortar with a pestle. It is likely that due to the water the outer layer of the tetracycline hydrochloride particles dissolves causing the particles to disperse.
12.5.1.2 The Function of Solid Excipients
Sometimes it is not clear whether an excipient is solely an excipient or has active substance properties.
In dermal bases indifferent solid substances may:
Act as filler material: powders for cutaneous application sometimes need a filler
Increase the consistency
Cause cooling and drying of the skin; finely divided solid excipients enlarge the surface of the skin resulting in increased evaporation of water and increased loss of warmth
Act as an adhesive; some solid excipients, such as talc result in an improved adherence of the preparation on the skin
Act as astringent; salts, aluminium oxide and zinc oxide cause the skin to astringe; the blood capillaries contract and slow down the exudation
Prevent agglomeration (anhydrous colloidal silica)
Apart from the general descriptions in Chap. 23, some details, also on function, are given on the most important solid excipients for cutaneous preparations:
Talc is a silicate often used in powders and suspensions for cutaneous application. It is a fine substance that consists mainly of magnesium silicate, but also contains some aluminium silicate. It has good flow properties and adheres well to the skin. It has a very low water absorbing capacity. A disadvantage of talc is the chance on formation of granulomas if it ends up in wounds. The combination of talc and zinc oxide in combination with water or other volatile solvents results in cooling of the skin. The cooling is the result of an increased surface area which allows water to evaporate more easily.
Zinc oxide is an astringent. It dries, cools and protects the skin and is weakly antibacterial. It may be used in nearly all types of cutaneous preparations. However, it is unsuitable for the hairy skin because it cannot be washed out.
Calamine is a mixture of zinc oxide, basic zinc carbonate and zinc silicate with a small amount of ferric oxide. Calamine gives a salmon colour to preparations.
Magnesium stearate and zinc stearate cool and cover the skin and in combination with fatty oil, such as arachis oil, they form magnesium oleate and zinc oleate. These oleates act as thickening agents and render the oil into oleogels (see Sect. 23.7.3 and Table 23.20).
Potato starch or rice starch is generally used as a component of cutaneous powders and pastes with a high amount of solids. Starch absorbs a high percentage of water. As a result it swells. This may lead to the formation of a crust on wounds. It is therefore not suitable for acute exsudative skin disorders.
12.5.2 Lipophilic Phase
The lipophilic phase consists of hydrophobic, oily semisolid and liquid substances. With regard to chemistry only triglycerides are defined as fats. Hydrophobic substances such as hydrocarbons, waxes and fatty alcohols are not fats. However, the hydrophobic phase is often named as ‘fat phase’. It covers the skin and prevents the evaporation of water. As a result the skin becomes hydrated. The degree of hydration depends on the properties of the hydrophobic components. Hydrophobic substances that do not penetrate the skin generally have a stronger hydrating effect as those that penetrate.
Hydrophobic substances are classified based on their structure:
12.5.2.1 Hydrocarbons (Paraffin Waxes)
Hydrocarbons such as liquid paraffin and white soft paraffin are also called mineral oils. They hardly penetrate the skin and in cutaneous preparations they are mainly used for protection of the surface of the skin or occlusion. They absorb little or no water. Because they cover the skin they prevent evaporation of water and hydrate the skin. Hydrocarbons are used in lipophilic creams, hydrophobic ointments and pastes.
12.5.2.2 Fatty Oils and Fats
In chemical terms fatty oils and fats are esters of glycerol and fatty acids. Arachidis oil and Miglyol 812 (medium chain triglycerides) are examples of often used fatty oils. In contrast to hydrocarbons they are biodegradable. They hardly absorb water but cover the skin less extensive than the hydrocarbons. Fatty oils and fats reduce the viscosity of semisolid cutaneous preparations and improve their spreadability. They are used in soft pastes for example.
12.5.2.3 Fatty Alcohols
Fatty alcohols such as cetostearyl alcohol and wool alcohols are lipophilic chains with a hydroxyl group at the end. Because of the combination of the lipophilic chain and the hydrophilic hydroxyl group these structures reduce the surface tension (see Sect. 18.4.3). Cetostearyl alcohol is a mixture of cetyl alcohol and stearyl alcohol. It is a weak water-in-oil emulsifying agent and it is a component of self-emulsifying waxes.
12.5.2.4 Waxes
Waxes such as bees wax, wool fat and decyl oleate are esters of fatty alcohols and fatty acids (see Sect. 23.3.5). The solid waxes are mainly used to improve the consistency of semi-solid preparations. Due to the presence of impurities such as fatty alcohols some waxes are weak w/o emulsifying agents. Although wool fat is classified as a wax it has different characteristics due to its ‘impurities’. Wool fat is extracted from the wool of sheep by water. It consists of a complex and variable mixture of several esters and polyesters of alcohols (wool fat alcohols) of high molecular weight and fatty acids. Wool fat is a stronger emulsifier than other waxes because of the presence of these esters. It can absorb water in a range up to 25 % of its weight. Hydrous wool fat (lanoline) is a w/o emulsion consisting of 75 % wool fat and 25 % water. Decyl oleate is a liquid and is used as a component of the lipophilic phase in o/w creams.
The liquid isopropyl myristate is closely related to the waxes. However it is not a wax as it is not an ester of a fatty alcohol and fatty acid. It has similar characteristics to fatty oils. It is mainly used to enhance the spreadability of oil-in-water emulsifying ointments.
12.5.3 Aqueous Phase
The aqueous phase may contain, apart from water, water-miscible liquids as humectants, co-solvents or penetration enhancers. The aqueous phase has to be preserved. If substances are dispersed in the aqueous phase they are considered part of the solid phase (see Sect. 12.5.1). For the substances reference is made to appropriate sections of Chap. 23 Raw materials. Just some functional details are mentioned here.
12.5.3.1 Water
The use of purified water is recommended to keep the initial contamination low and thereby to hold the Ph. Eur. requirements for microbiological quality of non-sterile pharmaceutical preparations. The requirements of the chemical and microbiological purity of purified water are well defined, see Sect. 23.3.1. The concentration of ions in purified water is low, which is an advantage as they may catalyse degradation of active substances and excipients and form complexes with active substances and excipients.
12.5.3.2 Co-solvents
Ethanol (see Sect. 23.3.2), propylene glycol (see Sect. 23.3.3) and glycerol (see Sect. 23.3.3) are often used as co-solvents (see Sect. 18.1.3).
The formula should allow hydrophilic active substances and excipients to dissolve completely in the aqueous phase. Information on solubility of substances can be found in several reference works [15, 17, 18]. However, the solubility in mixtures of solvents is only rarely to be found. It is not identical to the solubility in separate solvents because it is influenced by the interaction between the solvents.
12.5.3.3 Prevention of Water Loss (Humectants)
Water easily evaporates from warm skin. Due to evaporation a cutaneous preparation looses its characteristics. To prevent water loss humectants are added. Humectants are non-volatile solvents that prevent water loss during storage as well as after application to the skin. Examples are propylene glycol, glycerol 85 % and sorbitol 70 %. Humectants are often used in cutaneous suspensions, hydrophilic creams and hydrogels.
12.5.3.4 Viscosity Enhancement
Increasing the viscosity of the aqueous phase will result in the formation of a gel, which may be applied easier to the skin than water. In creams the increase of the viscosity may improve the physical stability of the emulsion, see Sect. 12.5.5. Carbomers 0.2–0.3 % (see Sect. 23.7.3.5) and cellulose derivates (see Sect. 23.7.3.2) are used for this purpose. A gel with carbomers is clear and leaves no residue on the skin. The disadvantage of carbomers is that the viscosity of the aqueous phase increases only at pH 6 or higher. Active substances that require a low pH, for example because of solubility or stability, are not compatible with carbomer gels. Additionally, the negative charge on the carbomer molecule causes many incompatibilities with cationic substances. Cellulose derivates show less incompatibility than carbomer but a disadvantage is the formation of a thin layer on the skin, a so called xerogel, after evaporation. This gives the patient a tightening sensation.
Enhancing the viscosity of an aqueous suspension is often necessary to obtain a reasonable physical stability (see Sect. 18.4.2.2). Apart from the already mentioned viscosity enhancers, also mineral viscosity enhancing substances, such as anhydrous colloidal silica (2–4 %, usually 2 %), and colloidal aluminium magnesium silicate (2–4 %, usually 2 %) or bentonite (1–2 %) are used. All percentages refer to the final amount of the preparation.
12.5.3.5 Preservation
Pharmaceutical preparations that contain water are susceptible of microbiological contamination. This applies specifically to preparations in which water is the outer phase (o/w emulsions). In hydrophilic emulsions microbiological contamination can easily spread throughout the whole preparation. Moreover, the outer phase has contact with the environment where microbiological contamination originates.
To ensure the microbiological quality of cutaneous preparations the following measures should be taken:
Ingredients should have a low initial microbiological contamination (see Sect. 23.1.7). Specifically ingredients of natural origin and water may contain relatively high amounts of micro-organisms.
The aqueous phase has to be preserved by the addition of preservatives or other substances with a preserving function. Many preservatives are sensitising; sorbic acid and methyl parahydroxybenzoate are commonly used. Consideration should be given to not using preservatives if the preparation is intended for large skin surfaces, in order to reduce the risk of sensitisation.
If the aqueous phase is emulsified in the lipophilic phase (w/o system) it is not always necessary to add a preservative. Theoretically, in w/o systems micro-organisms die inside the small water droplets due to a lack of oxygen and nutrients. If the initial contamination is low, if contamination during preparation is prevented and the microbiological quality is monitored at release, this theory may be applicable. If preservation is required in a w/o system propylene glycol could be added to the aqueous phase. Microbiological challenge tests for a w/o ‘cooling ointment’ (Table 12.31) showed that the addition of 10 % propylene glycol gave the formulation some preservative properties. The addition of 20 % would be even better, however in that formulation a compromise had to include improvement of microbiological stability without decreasing physical stability or increasing penetration too much.
Preservatives need to be hydrophilic as they act in the aqueous phase where micro-organisms live. To penetrate the cell wall of the micro-organism and interfere with the cell life cycle preservatives need to be hydrophobic as well. Because of the partly hydrophobic characteristics preservatives may diffuse into the lipophilic phase or will be solubilised in the aqueous phase. In this way the concentration of the preservative in the aqueous phase may become lower than required. Formulations of hydrophilic emulsions therefore will contain higher concentrations of preservatives than those of hydrophilic solutions.
Table 23.21 gives an overview of preservatives. For cutaneous preparations as said sorbic acid and methyl parahydroxybenzoate are mostly used. Antimicrobial properties of ethanol and propylene glycol are often made use of.
The activity of the preservative depends on the concentration in the aqueous phase and therefore on the partition coefficient (n-octanol/water). Because of its relatively favourable partition coefficient sorbic acid is a suitable preservative for o/w emulsions. Therefore it is often used in hydrophilic cream bases. Sorbic acid holds a carboxyl group that is deprotonated above pH 4–5. Sorbic acid only takes effect in the non-ionised form that means only in acidic solutions. However, at relatively low pH sorbic acid is degraded by oxidation. To prevent oxidation sorbic acid is often used in combination with potassium sorbate in order to obtain a pH value of 4–5.
Methyl hydroxybenzoate has an unfavourable partition coefficient. Therefore it is not a suitable preservative for o/w emulsions. It is mainly used in hydrogels. In some countries a combination with propyl hydroxybenzoate is used as ‘Preserved water’. A typical mixture contains 0.075 % of methyl parahydroxybenzoate and 0.025 % of propyl parahydroxybenzoate in purified water.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


