KEY CONCEPTS
![]() The attitude of the patient and sexual partner toward contraceptive methods, efficacy rate, the reliability of the patient in using the method correctly (which may affect the effectiveness of the method), noncontraceptive benefits, and the patient’s ability to pay must be considered when selecting a contraceptive method.
The attitude of the patient and sexual partner toward contraceptive methods, efficacy rate, the reliability of the patient in using the method correctly (which may affect the effectiveness of the method), noncontraceptive benefits, and the patient’s ability to pay must be considered when selecting a contraceptive method.
![]() Patient-specific factors (e.g., frequency of intercourse, age, smoking status, and concomitant diseases or medications) must be evaluated when selecting a contraceptive method.
Patient-specific factors (e.g., frequency of intercourse, age, smoking status, and concomitant diseases or medications) must be evaluated when selecting a contraceptive method.
![]() Adverse effects or difficulties using the chosen method should be monitored carefully and managed in consideration of patient-specific factors.
Adverse effects or difficulties using the chosen method should be monitored carefully and managed in consideration of patient-specific factors.
![]() Accurate and timely counseling on the optimal use of the contraceptive method and strategies for minimizing sexually transmitted diseases must be provided to all patients when contraceptives are initiated and on an ongoing basis.
Accurate and timely counseling on the optimal use of the contraceptive method and strategies for minimizing sexually transmitted diseases must be provided to all patients when contraceptives are initiated and on an ongoing basis.
![]() Emergency contraception may prevent pregnancy after unprotected intercourse or when regular contraceptive methods have failed.
Emergency contraception may prevent pregnancy after unprotected intercourse or when regular contraceptive methods have failed.
Unintended pregnancy is a significant public health problem. In the United States, approximately 6 million females become pregnant each year.1 The most recent data reveal that 37% of pregnancies are unintended, with the highest rates occurring in women aged 20 to 34 years.1 However, teen pregnancy rates are still an issue and slow to decline; teen births account for 11% of all the births in the United States.1 About half of all unintended pregnancies end in abortion, and 40% occur in sexually active couples who claim they used some method of contraception.1 If the goal of contraception—for pregnancies to be planned and desired—is to be realized, education on the use and efficacy of contraceptive methods must be improved.
ETIOLOGY AND PATHOPHYSIOLOGY
Comprehension of the hormonal regulation of the normal menstrual cycle is essential to understanding contraception in women (Fig. 62-1). The cycle of menstruation begins with menarche, usually around age 12 years, and continues to occur in nonpregnant women until menopause, usually around age 50 years. Factors such as race, body weight, medical conditions, and family history can affect the menstrual cycle.2 The cycle includes the vaginal discharge of sloughed endometrium called menses. The menstrual cycle comprises three phases: follicular (or preovulatory), ovulatory, and luteal (or postovulatory).
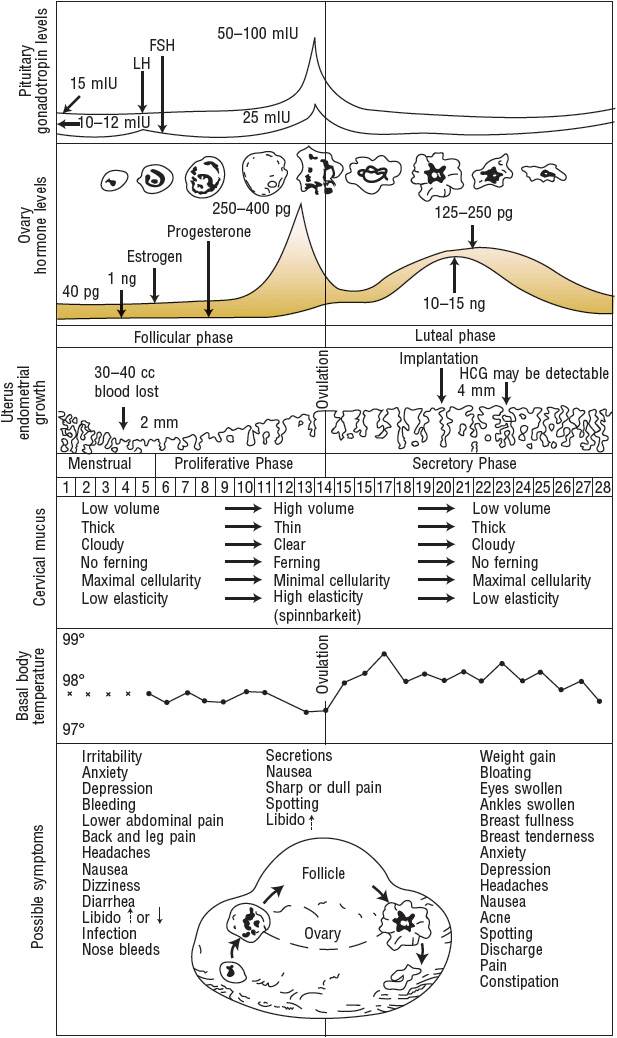
FIGURE 62-1 Menstrual cycle events, idealized 28-day cycle. (FSH, follicle-stimulating hormone; HCG, human chorionic gonadotropin, LH, luteinizing hormone.) LH: 15 milli-international units/mL = 15 international units/L; 50 to 100 international units/mL = 50 to 100 international units/L. FSH: 10 to 12 milli-international units/mL = 10 to 12 international units/L; 25 milli-international units/mL = 25 international units/L. Estrogen: 40 pg/mL = ∼150 pmol/L; 250 to 400 pg/mL = ∼920 to 1470 pmol/L; 125 to 250 pg/mL = ∼460 to 920 pmol/L. Progesterone: 1 ng/mL = 3 nmol/L; 10 to 15 ng/mL = ∼30 to 50 nmol/L. Temperatures: 99° F = 37.2° C; 98° F = 36.7° C; 97° F = 36.1° C.
(From Hatcher et al.2 This figure may be reproduced at no cost to the reader.)
The Menstrual Cycle
The first day of menses is referred to as day 1 of the menstrual cycle and marks the beginning of the follicular phase.2 The follicular phase continues until ovulation, which typically occurs on day 14. The time after ovulation is referred to as the luteal phase, which lasts until the beginning of the next menstrual cycle. The median menstrual cycle length is 28 days, but it can range from 21 to 40 days. Generally, variation in length is greatest in the follicular phase, particularly in the years immediately after menarche and before menopause.2
The menstrual cycle is influenced by the hormonal relationships among the hypothalamus, anterior pituitary, and ovaries.2 The hypothalamus secretes gonadotropin-releasing hormone (GnRH) in a pulsatile fashion.2 These GnRH bursts stimulate the anterior pituitary to secrete bursts of gonadotropins, follicle-stimulating hormone (FSH), and luteinizing hormone (LH). FSH and LH direct events in the ovarian follicles that result in the production of a fertile ovum.
Follicular Phase
In the first 4 days of the menstrual cycle, FSH levels rise and allow the recruitment of a small group of follicles for continued growth and development (Fig. 62-1).2 Between days 5 and 7, one follicle becomes dominant and later ruptures, releasing the oocyte. The dominant follicle develops increasing amounts of estradiol and inhibin, which cause a negative feedback on the hypothalamic secretion of GnRH and pituitary secretion of FSH, causing atresia of the remaining follicles recruited during the cycle.
Once the follicle has received FSH stimulation, it must receive continued FSH stimulation or it will die.2 FSH allows the follicle to enlarge and synthesize estradiol, progesterone, and androgen. Estradiol stops the menstrual flow from the previous cycle, thickening the endometrial lining of the uterus to prepare it for embryonic implantation. Estrogen is responsible for increased production of thin, watery cervical mucus, which will enhance sperm transport during fertilization. FSH regulates the aromatase enzymes that convert androgens to estrogens in the follicle. If a follicle has insufficient aromatase, the follicle will not survive.
Ovulation
When estradiol levels remain elevated for a sustained period of time, the pituitary releases a midcycle LH surge (Fig. 62-1).2 This LH surge stimulates the final stages of follicular maturation and ovulation (follicular rupture and release of the oocyte). On average, ovulation occurs 24 to 36 hours after the estradiol peak and 10 to 16 hours after the LH peak. The LH surge, which occurs 28 to 32 hours before a follicle ruptures, is the most clinically useful predictor of approaching ovulation. After ovulation, the oocyte is released and travels to the fallopian tube, where it can be fertilized and transported to the uterus for embryonic implantation. Conception is most successful when intercourse takes place from 2 days before ovulation to the day of ovulation.
Luteal Phase
After rupture of the follicle and release of the ovum, the remaining luteinized follicles become the corpus luteum, which synthesizes androgen, estrogen, and progesterone (Fig. 62-1).2 Progesterone helps to maintain the endometrial lining, which sustains the implanted embryo and maintains the pregnancy. It also inhibits GnRH and gonadotropin release, preventing the development of new follicles. If pregnancy occurs, human chorionic gonadotropin prevents regression of the corpus luteum and stimulates continued production of estrogen and progesterone secretion to maintain the pregnancy until the placenta is able to fulfill this role.
If fertilization or implantation does not occur, the corpus luteum degenerates, and progesterone production declines.2 The life span of the corpus luteum depends on the continuous presence of small amounts of LH, and its average duration of function is 9 to 11 days. As progesterone levels decline, endometrial shedding (menstruation) occurs, and a new menstrual cycle begins. At the end of the luteal phase, when estrogen and progesterone levels are low, FSH levels start to rise, and follicular recruitment for the next cycle begins.
EPIDEMIOLOGY
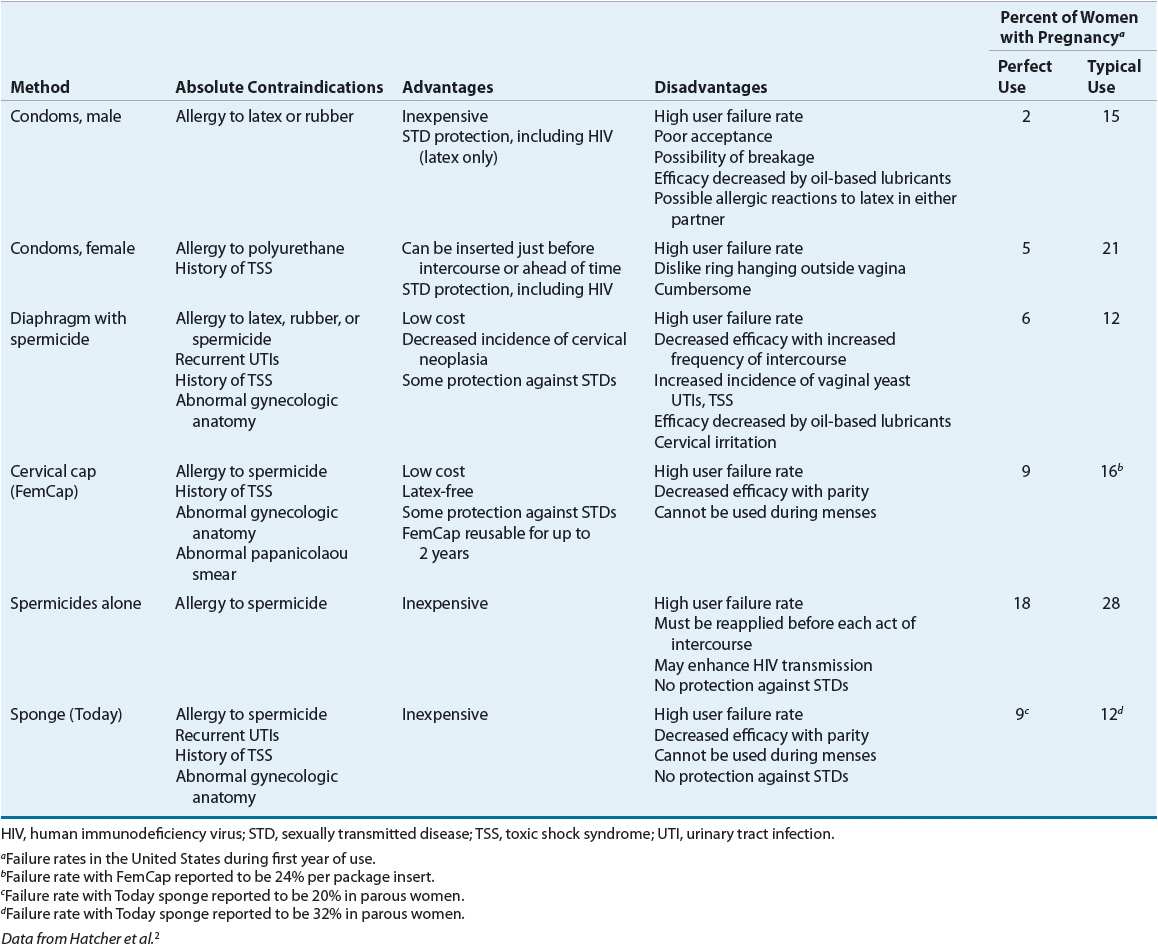
Contraception implies the prevention of pregnancy following sexual intercourse by inhibiting viable sperm from coming into contact with a mature ovum (i.e., methods that act as barriers or prevent ovulation) or by preventing a fertilized ovum from implanting successfully in the endometrium (i.e., mechanisms that create an unfavorable uterine environment). These methods differ in their relative effectiveness, safety, and patient acceptability (Tables 62-1 and 62-2).2,3
TABLE 62-1 Comparison of Unintended Pregnancy and Continuation Rates for Pharmacologic Contraceptive Methods
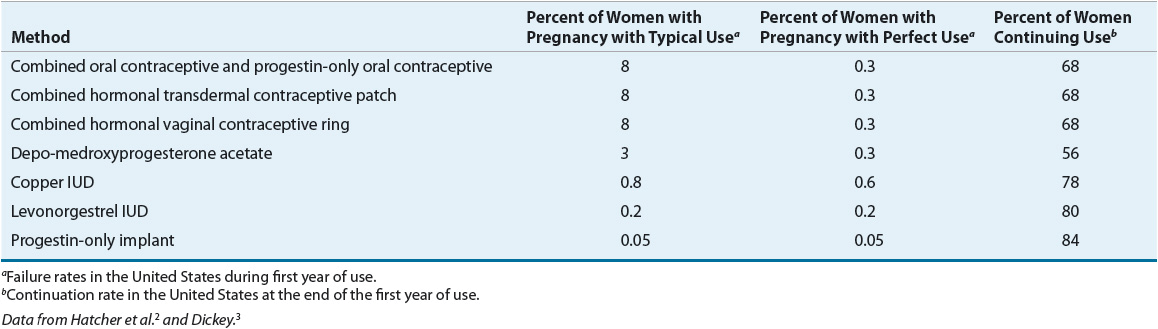
TABLE 62-2 Comparison of Methods of Nonhormonal Contraception
The actual effectiveness of any contraceptive method is difficult to determine because many factors affect contraceptive failure. A failure in patients who use the contraceptive agent properly is considered a method failure or perfect-use failure. It is also important to consider user failure or typical-use failure rates, which are usually higher because they take into account the user’s ability to follow directions correctly and consistently.2,3
CLINICAL PRESENTATION
Most health maintenance annual visits should include assessment of and counseling about reproductive health. Clinicians may use this opportunity to provide contraception and educate patients on prevention of sexually transmitted diseases (STDs). Traditionally, hormonal contraception is provided subsequent to breast and pelvic examinations. However, the need for the physical examination may delay access to contraception and reinforces the incorrect perception that these methods of contraceptives are harmful. Therefore, the American Congress of Obstetrics and Gynecology (ACOG) allow provision of hormonal contraception after a simple medical history and blood pressure measurement.4 Other preventive measures, such as pelvic and breast examinations, provision of the human papillomavirus vaccine, and screening for cervical neoplasia, can be accomplished during routine annual office visits.4,5
TREATMENT
Desired Outcomes
The obvious goal of treatment with all methods of contraception is to prevent pregnancy. However, many health benefits are associated with contraceptive methods, including prevention of STDs (with condoms), improvements in menstrual cycle regularity (with hormonal contraceptives), improvements in certain health conditions (with hormonal contraceptives), and management of perimenopause.2,6
Nonpharmacologic Therapy
Periodic Abstinence
![]()
![]() Motivated couples may use the abstinence (rhythm) method of contraception, avoiding sexual intercourse during the days of the menstrual cycle when conception is likely to occur. Physiologic changes, such as basal body temperature and cervical mucus, are used during each cycle to determine the fertile period. The major drawbacks are the relatively high pregnancy rates and avoidance of intercourse for several days during each menstrual cycle.2
Motivated couples may use the abstinence (rhythm) method of contraception, avoiding sexual intercourse during the days of the menstrual cycle when conception is likely to occur. Physiologic changes, such as basal body temperature and cervical mucus, are used during each cycle to determine the fertile period. The major drawbacks are the relatively high pregnancy rates and avoidance of intercourse for several days during each menstrual cycle.2
Barrier Techniques
![]()
![]() The effectiveness of barrier methods depends almost exclusively on motivation to use them consistently and correctly.2 These methods include condoms, diaphragms, cervical caps, and sponges (Table 62-2). A major disadvantage is higher failure rates than most hormonal contraceptives; thus, provision of counseling and an advanced prescription for emergency contraception (EC) are recommended for all patients using barrier methods as their primary means of contraception.
The effectiveness of barrier methods depends almost exclusively on motivation to use them consistently and correctly.2 These methods include condoms, diaphragms, cervical caps, and sponges (Table 62-2). A major disadvantage is higher failure rates than most hormonal contraceptives; thus, provision of counseling and an advanced prescription for emergency contraception (EC) are recommended for all patients using barrier methods as their primary means of contraception.
Male condoms create a mechanical barrier, preventing direct contact of the vagina with semen, genital lesions, and infectious secretions.2 Most condoms in the United States are made of latex, which is impermeable to viruses. A small proportion are made from lamb intestine, which is not impermeable to viruses. Synthetic condoms manufactured from polyurethane are another option; these condoms are latex-free and do protect against viruses. Condoms are used worldwide as protection from STDs including human immunodeficiency virus (HIV). When condoms are used in conjunction with any other barrier method, their effectiveness theoretically approaches 98%. Spillage of semen or perforation and tearing of the condom can occur, but proper use minimizes these problems. Mineral oil-based vaginal drug formulations (e.g., Cleocin, Premarin, and Monistat), lotions, or lubricants can decrease the barrier strength of latex, thus making water-soluble lubricants (e.g., Astroglide, K-Y Jelly) preferable. Condoms with spermicides are no longer recommended because they provide no additional protection against pregnancy or STDs and may increase vulnerability to HIV.
The female condom is a prelubricated, loose-fitting polyurethane sheath, closed at one end, with flexible rings at both ends.2 Properly positioned, the ring at the closed end covers the cervix, and the sheath lines the walls of the vagina. The outer ring remains outside the vagina, covering the labia. The pregnancy rate is reported to be higher when compared to male condoms. Male and female condoms should not be used together, as slippage and device displacement may occur.
The diaphragm, a reusable dome-shaped rubber cap with a flexible rim that is inserted vaginally, fits over the cervix in order to decrease access of sperm to the ovum. The diaphragm requires a prescription from a clinician who has fitted the patient for the correct size.2 Its efficacy is increased when it is used in conjunction with spermicidal cream or jelly. The diaphragm may be inserted up to 6 hours before intercourse and must be left in place for at least 6 hours afterward. However, leaving it in place for more than 24 hours is not recommended due to the potential for toxic shock syndrome (TSS). With subsequent acts of intercourse, the diaphragm should be left in place, and a condom should be used for additional protection.
The cervical cap (FemCap) is a soft, deep cup with a firm round rim that is smaller than a diaphragm and fits over the cervix like a thimble.2 The cervical cap is available in three sizes and requires a prescription from a clinician who has fitted the patient for the correct size. It should be filled with spermicide prior to insertion. The cervical cap can be inserted 6 hours prior to intercourse and should not be removed for at least 6 hours after intercourse. It can remain in place for multiple episodes of intercourse without adding more spermicide but should not be worn for more than 48 hours at a time to reduce the risk of TSS. Failure rates are higher than with other methods. Diaphragms and cervical caps do not protect against some STDs including HIV, thus condoms should also be used.
Pharmacologic Therapy
Spermicides
![]()
![]() Spermicides, most of which contain nonoxynol-9, are chemical surfactants that destroy sperm cell walls and act as barriers that prevent sperm from entering the cervical os.2 They are available as creams, films, foams, gels, suppositories, sponges, and tablets. Spermicides offer no protection against STDs. In fact, when used frequently (more than two times per day), nonoxynol-9 may increase the risk of transmission of HIV by causing small disruptions in the vaginal epithelium.2,7,8 The World Health Organization (WHO) and the Centers for Disease Control and Prevention (CDC) do not promote products containing nonoxynol-9 for protection against STDs.
Spermicides, most of which contain nonoxynol-9, are chemical surfactants that destroy sperm cell walls and act as barriers that prevent sperm from entering the cervical os.2 They are available as creams, films, foams, gels, suppositories, sponges, and tablets. Spermicides offer no protection against STDs. In fact, when used frequently (more than two times per day), nonoxynol-9 may increase the risk of transmission of HIV by causing small disruptions in the vaginal epithelium.2,7,8 The World Health Organization (WHO) and the Centers for Disease Control and Prevention (CDC) do not promote products containing nonoxynol-9 for protection against STDs.
Spermicide-Implanted Barrier Techniques
![]()
![]() The vaginal contraceptive sponge (Today) contains 1 g of the spermicide nonoxynol-9.2 It has a concave dimple on one side to fit over the cervix and a loop on the other side to facilitate removal. After being moistened with water, the sponge is inserted into the vagina up to 6 hours before intercourse. The sponge provides protection for 24 hours, regardless of the frequency of intercourse during this time. After intercourse, the sponge must be left in place for at least 6 hours before removal and should not be left in place for more than 24 to 30 hours to reduce the risk of TSS. Sponges should not be reused; after removal, they should be discarded. The sponge comes in one size and is available over the counter (OTC).
The vaginal contraceptive sponge (Today) contains 1 g of the spermicide nonoxynol-9.2 It has a concave dimple on one side to fit over the cervix and a loop on the other side to facilitate removal. After being moistened with water, the sponge is inserted into the vagina up to 6 hours before intercourse. The sponge provides protection for 24 hours, regardless of the frequency of intercourse during this time. After intercourse, the sponge must be left in place for at least 6 hours before removal and should not be left in place for more than 24 to 30 hours to reduce the risk of TSS. Sponges should not be reused; after removal, they should be discarded. The sponge comes in one size and is available over the counter (OTC).
Hormonal Contraception
Hormonal contraceptives contain a combination of estrogen and progestin or a progestin alone. Oral contraceptive (OC) preparations first became available in the 1960s, but options have expanded to include a transdermal patch, a vaginal contraceptive ring, and long-acting injectable, implantable, and intrauterine contraceptives.
Combined hormonal contraceptives (CHCs) contain both estrogen and progestin and work primarily before fertilization to prevent conception. Progestins provide most of the contraceptive effect by thickening cervical mucus to prevent sperm penetration, slowing tubal motility, delaying sperm transport, and inducing endometrial atrophy. Progestins block the LH surge, therefore inhibiting ovulation. Estrogens suppress FSH release from the pituitary, which may contribute to blocking the LH surge and preventing ovulation. However, the primary role of estrogen in hormonal contraceptives is to stabilize the endometrial lining and provide cycle control.2,3
Estrogens Two synthetic estrogens found in hormonal contraceptives available in the United States are ethinyl estradiol (EE) and mestranol. Mestranol must be converted by the liver to EE before it is pharmacologically active and is 50% less potent than EE.2,3 Most combined OCs, transdermal patch, and vaginal ring contain estrogen at doses of 20 to 50 mcg of EE.3
Progestins A variety of progestins are available in the United States, and they vary in their progestational activity and differ with respect to inherent estrogenic, antiestrogenic, and androgenic effects.2,3 Estrogenic and antiestrogenic properties are secondary to the extent of progestins’ metabolism to estrogenic substances. Androgenic activity depends on two variables: the presence of sex hormone (testosterone) binding globulin (SHBG-TBG) and the androgen-to-progesterone activity ratio. If the amount of SHBG-TBG is decreased, free testosterone levels increase, and androgenic side effects are more prominent.3
Considerations with Combined Hormonal Contraceptive Use ![]() When selecting a CHC, clinicians are challenged by weighing the benefits and risks associated with the many formulations available. The clinician must determine if the form of contraception is appropriate based upon the patient’s lifestyle and potential adherence. A complete medical examination and papanicolaou (Pap) smear are not necessary before a CHC is prescribed. A medical history and blood pressure measurement should be obtained before prescribing a CHC, along with a discussion of the benefits, risks, and adverse effects with each patient.2,3,9 For example, OCs are associated with noncontraceptive benefits, including relief from menstruation-related problems (e.g., decreased menstrual cramps, decreased ovulatory pain [mittelschmerz], and decreased menstrual blood loss), improvement in menstrual regularity, and decreased iron deficiency anemia.6 Women who take combination OCs have a reduced risk of ovarian and endometrial cancer. There is a 50% reduction in risk in women who have used OCs for 5 years or more, and protection may persist for more than 10 years post-use. Combination OCs may also reduce the risk of ovarian cysts, ectopic pregnancy, pelvic inflammatory disease, endometriosis, uterine fibroids, and benign breast disease. The CHC transdermal patch and vaginal ring are other combined hormonal options that may be more convenient for women than taking a tablet each day.
When selecting a CHC, clinicians are challenged by weighing the benefits and risks associated with the many formulations available. The clinician must determine if the form of contraception is appropriate based upon the patient’s lifestyle and potential adherence. A complete medical examination and papanicolaou (Pap) smear are not necessary before a CHC is prescribed. A medical history and blood pressure measurement should be obtained before prescribing a CHC, along with a discussion of the benefits, risks, and adverse effects with each patient.2,3,9 For example, OCs are associated with noncontraceptive benefits, including relief from menstruation-related problems (e.g., decreased menstrual cramps, decreased ovulatory pain [mittelschmerz], and decreased menstrual blood loss), improvement in menstrual regularity, and decreased iron deficiency anemia.6 Women who take combination OCs have a reduced risk of ovarian and endometrial cancer. There is a 50% reduction in risk in women who have used OCs for 5 years or more, and protection may persist for more than 10 years post-use. Combination OCs may also reduce the risk of ovarian cysts, ectopic pregnancy, pelvic inflammatory disease, endometriosis, uterine fibroids, and benign breast disease. The CHC transdermal patch and vaginal ring are other combined hormonal options that may be more convenient for women than taking a tablet each day.
![]()
![]() Adverse effects may hinder adherence and therefore efficacy, so they should be discussed prior to initiating a hormonal contraceptive agent.10 Excessive or deficient amounts of estrogen and progestin are related to the most common adverse effects.2,3,10 The CHC vaginal ring may be uncomfortable for some women and cause vaginal discharge. The CHC patch may cause irritation and now has information added to product labeling regarding the increased potential for thromboembolism.
Adverse effects may hinder adherence and therefore efficacy, so they should be discussed prior to initiating a hormonal contraceptive agent.10 Excessive or deficient amounts of estrogen and progestin are related to the most common adverse effects.2,3,10 The CHC vaginal ring may be uncomfortable for some women and cause vaginal discharge. The CHC patch may cause irritation and now has information added to product labeling regarding the increased potential for thromboembolism.
An important concern regarding the use of CHCs is the lack of protection against STDs. Because of their high efficacy in preventing pregnancy, patients may choose not to use condoms. In addition to public health awareness, clinicians must encourage patients to use condoms for prevention of STDs. OCs have an extensive history of safety concerns, which traditionally were related to high dose estrogen tablets. To replace the traditional absolute and relative contraindications to the use of OCs, the CDC developed a graded list of precautions for clinicians to consider when initiating CHCs (Table 62-3).2,9
TABLE 62-3 U.S. Medical Eligibility Criteria for Contraceptive Use: Classifications for Combined Hormonal Contraceptives
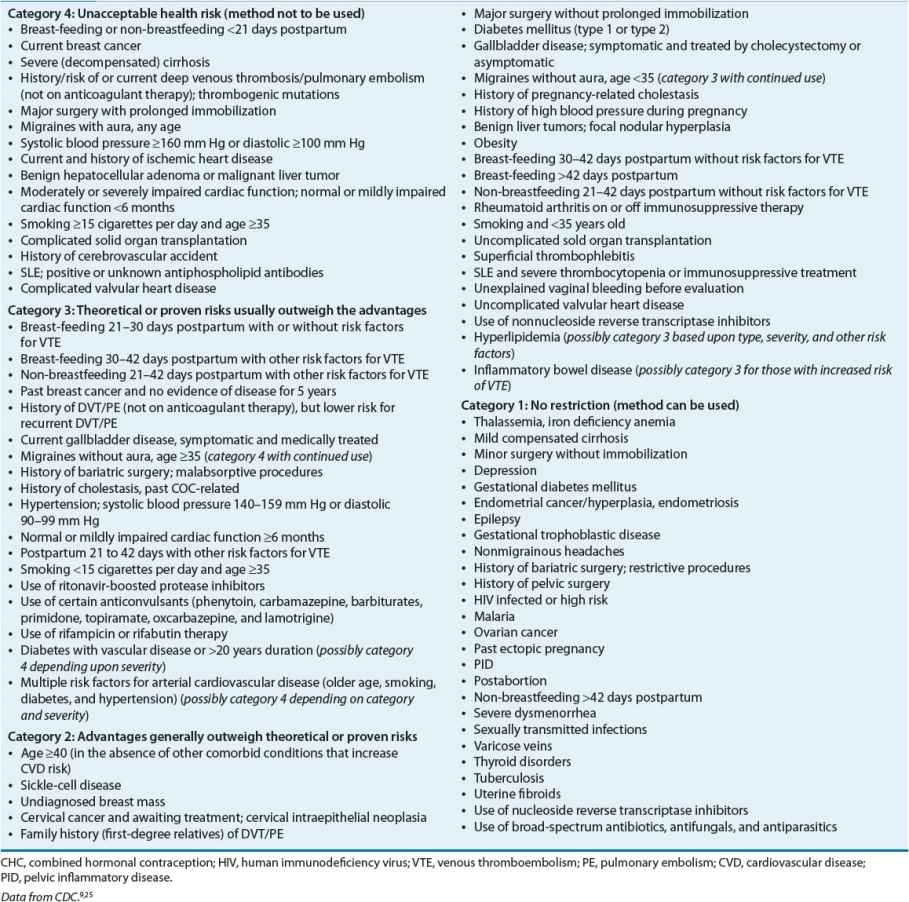
In addition to the CDC, the ACOG provides information for clinicians to use when selecting CHCs for women with coexisting medical conditions.11 Overall, the health risks associated with pregnancy, the specific health risks associated with CHCs, and the noncontraceptive benefits of CHCs should be factored into risk-to-benefit considerations.
Women Older Than 35 Years Use of CHC in women older than 35 is controversial. Older women, especially women in their 40s, retain a level of fertility even in the perimenopausal state and should use contraception to prevent pregnancy. Formulations with lower doses of estrogen (less than 30 mcg) have increased the use of CHCs in these women. In addition to the benefit of pregnancy prevention, they may improve or decrease the chance of developing perimenopausal and menopausal symptoms and increase bone mineral density (BMD). However, the benefits of using CHCs must be weighed against the risks in women older than 35. The increased risk of cardiovascular disease and venous thromboembolism (VTE) should be considered especially in perimenopausal women greater than 40. Older data suggest an increased risk of myocardial infarction (MI) in older women using CHCs, although many women in these studies were current smokers and used older formulations containing higher doses (greater than 50 mcg) of estrogen. More recent data do not support the increased risk of cardiovascular disease when low-dose formulations of CHCs are used in healthy, non-obese women. Other concerns include the increased risk of ischemic stroke in women with migraines and the increased risk of breast cancer in older women.9,11,12
The risks and benefits of using CHCs in women greater than 35 must be considered on an individual basis. It is recommended that use of CHCs (with less than 50 mcg of estrogen) may be considered in healthy nonsmoking women. CHCs should not be recommended in women older than 35 years with migraine (with or without aura), uncontrolled hypertension, smoking, or diabetes with vascular disease.9,11
Smoking In early studies, OCs with 50 mcg EE or more were associated with MI in women who smoked cigarettes.2,3,9,11 The United States case–control studies have found that both nonsmoking and smoking women, regardless of age, taking OCs with less than 50 mcg EE did not have an increased risk of MI or stroke. However, these studies included few women older than 35 years who were smokers. European studies, with a higher population of older smoking women, demonstrated an increased risk of MI in this population. Therefore, practitioners should prescribe CHC with caution, if at all, to women older than 35 years who smoke. Smoking 15 or more cigarettes per day by women in this age group is a contraindication to CHC, and the risks generally outweigh the benefits of CHC in those who smoke fewer than 15 cigarettes per day.9 Progestin-only contraceptive methods should be considered for women in this group.
Hypertension CHCs can cause small increases (i.e., 6 to 8 mm Hg) in blood pressure, regardless of estrogen dosage.3,9,11 This has been documented in both normotensive and mildly hypertensive women given a 30 mcg EE OC. In case–control studies of women with hypertension, OCs have been associated with an increased risk of MI and stroke. Use of low-dose CHC is acceptable in women younger than 35 years with well-controlled and frequently monitored hypertension. If a CHC-related increase in blood pressure occurs, discontinuing the CHC usually restores blood pressure to pretreatment values within 3 to 6 months.3 Systolic blood pressure greater than or equal to 160 mm Hg or diastolic blood pressure greater than or equal to 100 mm Hg is considered a contraindication to the use of CHCs. Hypertensive women who have end-organ vascular disease or who smoke should not use CHCs. Women with hypertension who are taking potassium-sparing diuretics, angiotensin-converting enzyme inhibitors, angiotensin-receptor blockers, or aldosterone antagonists may have increased serum potassium concentrations if they are also using an OC-containing drospirenone, which has antialdosterone properties.3
Dyslipidemia Generally, synthetic progestins adversely affect lipid metabolism by decreasing high-density lipoprotein (HDL) and increasing low-density lipoprotein (LDL).3,11 Estrogens tend to have more beneficial effects by enhancing removal of LDL and increasing HDL levels. Estrogens may moderately increase triglycerides. As a net result, most low-dose CHCs have no significant impact on HDL, LDL, triglycerides, or total cholesterol. CHCs containing more androgenic progestins (e.g., levonorgestrel) may result in lower HDL levels in some patients. Although the lipid effects of CHCs theoretically can influence cardiovascular risk, the mechanism of increased cardiovascular disease in CHC users is believed to be due to thromboembolic and thrombotic changes, not atherosclerosis. Women with controlled dyslipidemia can use low-dose CHCs, although periodic fasting lipid profiles are recommended. Women with uncontrolled dyslipidemia (LDL greater than 160 mg/dL [4.14 mmol/L], HDL less than 35 mg/dL [0.91 mmol/L], triglycerides greater than 250 mg/dL [2.83 mmol/L]) and additional risk factors (e.g., coronary artery disease, diabetes, hypertension, smoking, or positive family history) should consider an alternative method of contraception.
Diabetes Any effect of CHCs on carbohydrate metabolism is thought to be due to the progestin component.3,11 However, with the exception of some levonorgestrel-containing products, formulations containing low doses of progestins do not significantly alter insulin, glucose, or glucagon release after a glucose load in healthy women or in those with a history of gestational diabetes. The new progestins are believed to have little, if any, effect on carbohydrate metabolism. CHCs do not appear to alter the hemoglobin A1c values or accelerate the development of microvascular complications in women with diabetes. Therefore, nonsmoking women younger than 35 years with diabetes but no associated vascular disease can safely use CHCs. Diabetic women with vascular disease (e.g., nephropathy, retinopathy, neuropathy, or other vascular disease) or diabetes of more than 20 years’ duration should not use CHCs.9
Migraine Headaches Women with migraine headaches may experience a decreased or an increased frequency of migraine headaches when using CHCs.3,9,11,13 Studies have demonstrated a higher risk of stroke in women experiencing migraine with aura compared to women with simple migraine. In population-based studies, the risk of stroke in women with migraines has been elevated twofold to threefold. However, given the low absolute risk of stroke in young women (age less than 35 years), the ACOG recommends considering CHCs in healthy, nonsmoking women with migraine headaches without aura.11 Women of any age who have migraine with aura and women over the age of 35 with any type of migraine should not use CHC. Women who develop migraines (with or without aura) while receiving CHC should discontinue use and consider a progestin-only option.
Breast Cancer Worldwide epidemiologic data from 54 studies in 25 countries (many of which studied high dose OCs) were collected to assess the relationship between OCs and breast cancer.11 Overall, investigators noted a small increased risk of breast cancer associated with current or recent use, but OCs did not further increase risk in women with a history of benign breast disease or a family history of breast cancer. A more recent U.S.-based case–control study found no association between overall breast cancer and current or past OC use.11 This study also found no association between depo-medroxyprogesterone acetate (DMPA) and breast cancer. Although some studies have found differences in risk of breast cancer based on the presence of BRCA1 and BRCA2 mutations, the most recent cohort study found no association with low-dose OCs and the presence of either mutation. The choice to use CHCs should not be affected by the presence of benign breast disease or a family history of breast cancer with either mutation. Women with current or past history of breast cancer should not use CHCs.2,9
Thromboembolism Estrogens increase hepatic production of factor VII, factor X, and fibrinogen in the coagulation cascade, therefore increasing the risk of thromboembolic events (deep vein thrombosis, pulmonary embolism). These risks are increased in women who have underlying hypercoagulable states (e.g., deficiencies in antithrombin III, protein C, and protein S; factor V Leiden mutations, prothrombin G2010 A mutations) or who have acquired conditions (e.g., obesity, pregnancy, immobility, trauma, surgery, and certain malignancies) that predispose them to coagulation abnormalities.3,9,11 In the U.S. case–control studies, the risk of VTE in women currently using low-dose OCs (less than 50 mcg EE) was four times the risk in nonusers.3,11 However, this risk is less than the risk of VTE incurred during pregnancy. OCs containing newer progestins such as drosperinone, desogestrel, and norgestimate are associated with a slightly increased risk of thrombosis.2 Although the mechanism for this increased risk is unclear, it is thought that third- and fourth-generation progestins have a greater effect on the procoagulant, anticoagulant, and fibrinolytic pathways.2,14.15 These progestins may also be associated with increased resistance to protein C and may increase levels of sex hormone-binding globulin.2,14 It is thought that continuous, higher exposure to estrogen seen with the transdermal patch or vaginal ring is the reason for an increased thromboembolic risk with these agents as well. An advisory committee to the FDA decided to change the product labeling of the transdermal patch as well as products containing drosperinone to include additional information about the increased risk of thromboembolism.16,17 Therefore, for women who are at an increased risk of thromboembolism (e.g., older than 35 years, obesity, smoking, personal or family history of venous thrombosis, prolonged immobilization), it would be prudent to first consider low-dose oral estrogen contraceptives containing older progestins or progestin-only contraceptive methods. It is important to note that EC has not been associated with an increased risk of thromboembolic events.



