Chapter Fifteen. Congenital defects
Introduction
Congenital defects which are present at birth may be visible, involve obvious changes in organs or be hidden such as changes in protein molecules, for example haemoglobin or cell receptors. Moore & Persaud (2008) outline four clinically significant types of congenital anomalies:
• Malformation: A morphologic defect of an organ, part of an organ or larger region of the body that results from an intrinsically abnormal developmental process. Intrinsic implies that the developmental potential is abnormal from the beginning such as a chromosomal abnormality of a gamete at fertilisation.
• Disruption: A morphological defect of an organ, part of an organ or larger region of the body that results from extrinsic breakdown or interference with an originally normal developmental process. Environmental causes such as teratogens, for example drugs and viruses, should be considered.
• Deformation: An abnormal form, shape or position of a body part resulting from mechanical causes such as in oligohydramnios.
• Dysplasia: An abnormal organisation of cells into tissues because of dyshistogenesis or cellular disturbance.
Congenital defects occur in 2–3% of all live births and account for most severe illness during infancy and childhood and 25% of childhood deaths. However, most are minor and of no functional significance. The percentage of diagnosis increases as children develop and reaches 6% in 2-year-olds and 8% in 5-year-olds (Moore & Persaud 2008).
General causes of congenital abnormalities
The major causes of congenital abnormalities are (Carlson 2004):
• Unknown cause: 50%
• Multifactorial (genetic + environment): 25%
• Chromosomal anomaly: 10%
• Single gene defect: 8%
• Major environmental cause: 7%.
Genes
An introduction to genetics, including causation of fetal abnormalities, is presented in Chapter 3 and the role of genes in embryogenesis is found in Chapter 8. About 18% of fetal anomalies are caused by chromosomal and genetic factors. Mutations may occur in the DNA of protein coding genes or in the homeobox regulatory genes.
Teratogens
Teratology is the study of the causes, mechanisms and patterns of abnormal fetal development. Gregg’s finding in 1941 that rubella virus caused a syndrome of abnormalities (Ch. 14) was the first clear evidence of teratogenesis, and the terrible effects of the drug thalidomide between 1957 and 1962 convinced scientists of the environmental effect on a fetus that up until the 1940s had been thought to be totally protected from the outside world. Teratogens reach the fetus by crossing the placenta and causing DNA mutations.
Malformation of a particular structure is usually caused only during the sensitive period of its development (Moore & Persaud 2008), and the earlier a teratogen is present the more generalised the effect. If malformations occur in the first 17 days when the germ layers are being formed they are usually so complex as to be fatal. Between 3 and 8 weeks survival with major defect is likely. Following completion of organogenesis their effects are greatly reduced except in the brain and sensory organs where cell differentiation continues. Later in pregnancy some teratogens, especially micro-organisms may destroy already formed tissues.
Environmental and genetic interaction
The rate of new mutations can be increased by environmental factors such as microbial, biochemical and dietary factors, smoking, alcohol ingestion, radiation and many chemicals. These may interfere with embryonic development at very precise times during organogenesis. Some factors such as the rubella virus are easily associated, but others, such as environmental pollution, are more difficult to ascertain. These interactions are discussed in Chapter 8.
Drugs
Medicinal drugs may be dispensed by practitioners or bought over the counter from a chemist. Recreational drugs such as cocaine and heroine may be bought illegally on the streets. These drugs may damage gametes or may prevent nutrient absorption such that essential nutrients are absent at crucial times (Table 15.1). Taking medicinal drugs is common in pregnancy and several studies have shown that pregnant women may take up to four types of drug during their pregnancy, 50% of these in the first trimester (Moore & Persaud 2008).
| Drug | Effect |
|---|---|
| Thalidomide | Limb deformities, heart defects |
| Warfarin | Limb defects, central nervous system defects, retarded growth |
| Corticosteroids | Cleft palate and congenital cataract |
| Anticonvulsants such as phenytoin | Lip and palate deformities, mental retardation |
| Androgens | Masculinisation in female fetus |
| Oestrogens | Testicular atrophy in male |
| Diethylstilbestrol | Vaginal and cervical cancer at puberty |
| Cytotoxic drugs (especially folic acid antagonists) | Neural tube defects, cleft palate |
| Tetracycline | Staining of bones and teeth, thin tooth enamel, impaired bone growth |
Thalidomide, a drug taken for morning sickness around 1960, caused major limb reduction deformities and other problems (Fig. 15.1). The effects of taking thalidomide in early pregnancy are still being seen in South America where the drug is prescribed for leprosy. While care is taken to avoid prescribing it to pregnant women, people offer their drugs to friends and relatives, some of whom will be pregnant.
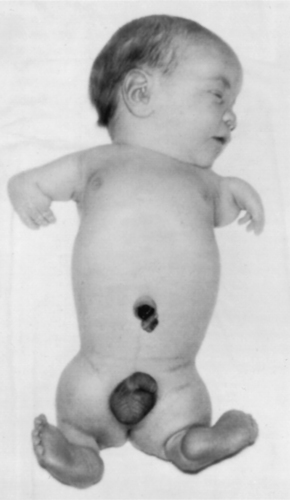 |
| Figure 15.1 Newborn male infant showing typical malformed limbs (meromelia—limb reduction) caused by thalidomide ingested by his mother during the critical period of limb development. (Reproduced with permission from Moore 1963.) |
Prenatal screening for congenital defects
In an ideal world congenital defects could be prevented. However, most women are seen for the first time when their pregnancies are already underway. Preconception advice (Ch. 8) will help to reduce the number of abnormal embryos conceived. Early diagnosis is essential so that a couple can be offered the choice of termination of pregnancy. Many defects can now be identified in specific populations. Techniques include ultrasonography, chorionic villus sampling, amniocentesis and maternal serum screening.
Turnpenny & Ellard (2007) discuss the above techniques and how they may revolutionise the diagnosis of embryonic and fetal malformations and genetic disease. However, Abramsky & Chapple (2003) consider the human side of prenatal screening and late pregnancy diagnosis of fetal abnormality, including parents’ own perspective. Medical technology may have raced ahead with little consideration of the ethical, legal and emotional dilemmas raised by an increased ability to detect fetal defects. Couples may not want to terminate the pregnancy and early diagnosis offers a chance to plan for the baby’s treatment after birth.
Ultrasonography
Most women are offered an ultrasound scan (USS) in pregnancy. The embryonic sac can be seen as early as 6 weeks following conception. USS screening is probably best carried out at 18–20 weeks’ gestation so that an accurate fetal age can be confirmed. Most obstetricians feel the advantages of routine screening far outweigh the disadvantages. Sullivan & Kirk (2003) give a very detailed account of USS by trimester and also discuss women’s experiences and attitudes to scanning.
How ultrasound works
Ultrasound imaging depends on the differences in tissue structure between organs. Sounds at a very high pitch (frequency of 3–10 MHz) are produced by a transducer. Because of its frequency, high-pitched sound travels in a narrow beam. These sound waves pass into the body until they reach a tissue and are reflected back. The sound echoes are detected electronically and transmitted onto the screen as a dot. The more dense the tissue, the stronger the echo and the whiter the visual display. Weaker echoes produce various shades of grey. Fluid-filled areas reflect no echoes, producing a black area.
Various types of display modes are used, each with its advantages. M-mode ultrasonography shows changes in a structure with time. B-mode ultrasonography shows the anatomy of a two-dimensional plane of scanning and can be used in real time. Doppler ultrasonography produces flow pattern information of the heart and blood vessels. With endosonography an endoscope can be inserted into the vagina to bring it closer to the fetus and receive a higher-resolution image. Real-time B-mode ultrasonography is most often used. Common defects diagnosed by mid-trimester fetal anomaly ultrasound include:
• Anencepahaly, microcephaly and hydrocephaly.
• Neural tube defects.
• Gastrointestinal defects such as atresia or omphalocele.
• Renal agenesis and polycystic kidneys.
• Body defects associated with chromosomal defects.
Obtaining fetal tissue for genetic testing
All invasive techniques carry a risk of infection, haemorrhage and fetal loss. The risks must be weighed against the likelihood of fetal abnormality being present.
Cells obtained by amniocentesis or chorionic villus sampling can be used for karyotyping for chromosomal abnormalities such as Down syndrome, genetic analysis using gene probes as in cystic fibrosis and sexing the embryo if there is a family history of X-linked disorders such as Duchenne muscular dystrophy. Enzyme assay for detection of inborn errors of metabolism is available in many disorders. These invasive tests should be carried out at a specialist centre.
Amniocentesis
A sample of amniotic fluid is withdrawn from the amniotic cavity through a transabdominal needle using ultrasound to avoid the placenta. This is usually carried out at 14–16 weeks when sufficient amniotic fluid is present. If carried out earlier the small amount of fluid present makes it difficult and there may be insufficient cells obtained to study. A further problem is that the desquamated cells (those shed by the fetus into the amniotic fluid) obtained are difficult to culture and it may take up to 3 weeks before the chromosomes can be counted. There is a 1% chance of miscarriage and the long waiting period for the results is distressing to the parents.
Chorionic villus sampling
Chorionic villus sampling




Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


