Chapter Fourteen. Common fetal problems
Introduction
This chapter covers a variety of topics concerning fetal danger. Although there is a blurred line between concepts applied to the fetus and the neonate, this chapter is concerned with the fetus. The following problems are discussed in relation to the neonate in Chapter 48, Chapter 49, Chapter 50, Chapter 51, Chapter 52 and Chapter 53.
Intrauterine growth retardation
Before discussing intrauterine growth retardation (IUGR), it is useful to understand some definitions:
• A low-birth-weight baby (LBW) is a baby who weighs 2500 g or less at birth.
• A very-low-birth-weight baby (VLBW) is a baby who weighs less than 1500 g at birth.
• A light-for-dates or small-for-gestational-age (SGA) baby is one whose birth weight is below the 10th centile for its gestational age but is not necessarily growth restricted.
• A large-for-gestational-age (LGA) baby is one whose birth weight is above the 90th centile for gestational age.
• A preterm infant is a baby born before 37 completed weeks of pregnancy (from LMP) irrespective of the birth weight. A preterm infant may also be SGA or LGA.
There are two categories of fetuses which appear SGA:
1. The fetus shows early departure from normal limits of growth that continues until delivery and is small because of conditions such as chromosomal abnormality or intrauterine infection.
2. The fetus shows arrest of previously normal growth caused by a factor outside the fetus such as maternal disease or placental pathology.
The term IUGR should be used only for the second category where growth is known to be arrested. Wrong classification of IUGR does not allow for fetuses that are small and healthy. This may lead to inappropriate interference in the course of pregnancy.
Complications of IUGR
In the antepartum and intrapartum periods there is an increase in the number of stillbirths, oligohydramnios and fetal distress. Neonatal complications including meconium aspiration syndrome, persistent fetal circulation, hypoglycaemia, hypocalcaemia, hyperviscosity syndrome and poor temperature control will be discussed in Section 4A of the book.
Factors adversely affecting fetal growth
Fetal growth depends on interacting factors such as genetic determinants, maternal health and nutrition, availability of growth substrates and an effective maternal blood supply to the placenta (Blackburn 2007). The essential substrates are oxygen, glucose and amino acids. Any decrease in substrate availability due to pathological conditions affecting mother, placenta and fetus will result in poor growth.
Fetal growth retardation may be asymmetric or symmetric. Asymmetric growth is when fetal weight is reduced out of proportion to length and head circumference and the fetus has little subcutaneous fat. The factors involved are usually maternal in origin. Symmetric growth retardation is due either to a congenital or genetic defect or to decreased growth potential in the fetus. The babies have the normal amount of subcutaneous fat for their size and the head circumference and length are in proportion to the weight.
Maternal conditions are:
• Hypertension.
• Chronic renal disease.
• Diabetes mellitus with vascular lesions.
• Sickle cell anaemia.
• Severe cardiac disease.
• Severe malnutrition.
• Smoking.
• Alcohol ingestion.
Fetoplacental problems include:
• Chromosomal abnormalities.
• Intrauterine infections.
• A history of a previous growth-retarded fetus.
• Multiple pregnancy.
• Small placental site and inadequate changes in uterine spiral arteries.
• Placental infarcts.
• Placenta praevia.
Maternal malnutrition
Although maternal malnutrition and low weight gain in pregnancy have been weakly associated with IUGR (Ott 2001) this is more likely to occur in developing countries. Severe protein calorie malnutrition, especially in the second half of pregnancy, will reduce birth weight; babies born in the Dutch famine of 1944–45 had a reduced birth weight in proportion to their length (Gluckman & Hanson 2005).
Smoking
Smoking is a prime cause of IUGR (Ott 2001). Tobacco smoking affects the fetoplacental unit in all three trimesters. Cigarette smoke contains many toxins which can affect cell proliferation and differentiation, in particular nicotine. Increased risk of miscarriage, fetal growth retardation, stillbirth, preterm birth and placental abruption has been reported (Jauniaux & Burton 2007). Also, carboxyhaemoglobin causes a sustained reduction in oxygen to the fetus with a prolonged effect on growth (Longo 1977). Birth weight reduction is between 120 and 430 g.
Alcohol consumption
Fetal alcohol syndrome (FAS) was first recognised in America. Alcohol has a low molecular weight and crosses the placental barrier. It is a known teratogen and fetal damage has been associated with any drinking. It is not possible to state how much alcohol causes the abnormalities associated with FAS so it is wise to abstain from drinking any alcohol during pregnancy. In a few women alcohol-induced malnutrition will add to the fetal problems.
The characteristic features of FAS are not all present in a particular infant:
• Deficient overall growth.
• Facial abnormalities: small eyes with inner epicanthic folds; poorly formed nasal bridge giving the nose a retroussé appearance; poor or absent vertical groove in a narrow top lip; ears that are large and simple in formation; and cleft palate.
• Musculoskeletal abnormalities: congenital hip lesions and thoracic cage abnormalities.
• Genitourinary abnormalities: undescended testes, male urethral abnormalities, hypoplastic labia in females; and kidney abnormalities.
• Cardiac abnormalities are common, mainly atrial or ventricular septal defects.
• Poor coordination of movement and learning difficulties.
• Alcohol withdrawal symptoms.
Placental insufficiency
In placental insufficiency the placenta is usually small with a reduction in the number of stem and villous capillaries and reduced uteroplacental blood flow with incomplete penetration by trophoblastic cells (Robinson et al 2000). There appears to be a failure in vascular invasion of the myometrial spiral arteries with increased risk of morbidity and mortality. These findings are also present in maternal conditions such as pre-eclampsia (Lyall & Robson 2000).
Multiple pregnancy
Poor fetal growth occurs in about 21% of twins, mainly due to abnormal placentation (see Ch. 13).
Genetic factors and chromosomal aberrations
The prevalence of genetic and chromosome disorders amongst babies with IUGR is higher than normal with an incidence of congenital abnormalities of between 5% and 27% compared to 0.1–4% in babies with normal growth (Ott 2001). It is particularly common in babies with trisomies such as Down syndrome. The majority of these babies have symmetric growth retardation.
Diagnosis and management of IUGR
The first step in managing IUGR is to identify those women at risk, then to differentiate the small, healthy babies from those with genuine IUGR (Robinson et al 2000). Monitoring at-risk fetuses allows management decisions to be made. Using a combination of clinical and ultrasound methods should enable a diagnosis to be made in 95% of cases.
Ultrasound
Ott (2006) stated that a combination of Doppler velocity wave form analysis of fetal vessels and ultrasonic estimation of fetal weight by measuring abdominal circumference appears to be the best method of identifying and evaluating IUGR. Soregaroli et al (2002) found a correlation between umbilical Doppler velocimetry abnormalities and an increased incidence of perinatal complications. The more abnormal the umbilical arterial blood flow, the more the fetal risk.
Measuring fetal subcutaneous fat
The research of Gardeil et al (2000) included 137 pregnant women. They found a correlation between babies with less than 5 mm of subcutaneous fat at 38 weeks and a low height:weight ratio ( ponderal index). There were more instances of neonatal morbidity in those babies than in the control group with a subcutaneous fat measurement greater than 5 mm.
Other tests of placental function
Many tests have been shown to be of no value in assessment of placental function and are of historical value only. These include serial estriol estimation, human placental lactogen assays, contraction cardiotocography (CTG) stress testing and monitoring daily fetal movements. Measuring serial fundal height is of value in selecting women for referral to ultrasound screening. Doppler assessment combined with non-stress CTG may be important in diagnosing and managing IUGR (Ott 2006).
Fetal biophysical profile
Four fetal variables are combined to try to reduce the incidence of false-positive diagnoses. These are fetal movement, tone, breathing movements and amniotic fluid volume. In addition a 20-min CTG is carried out. Lalor et al (2007) reviewed five trials involving 2974 women with high-risk pregnancies and found no reduction either in the number of babies who died or in those with low Apgar scores. However, fetal biophysical profile was associated with a significant increase in induction and caesarean section. They suggest more evaluation of the method is needed.
Delivery of the baby
Once fetal lung maturity is achieved and, preferably after 34 weeks, the baby should be delivered. Excluding congenital malformations, intrapartum asphyxia is a major cause of morbidity and mortality. There is an increased risk of low 5-min Apgar scores and later neurological deficits. Asphyxia must be prevented:
• Direct fetal monitoring by scalp electrode.
• Care with analgesia and epidural anaesthesia may be best.
• The second stage of labour should be short and low forceps used if necessary.
• A paediatrician should be present at delivery.
Most of these babies now survive the neonatal period but they remain smaller than their age cohorts for several years. A major concern is that chronic intrauterine malnutrition may lead to a permanent decrease in brain cells. Small size at birth and low ponderal index are linked to coronary heart disease, high blood pressure and diabetes in later life (Barker 1998, Gluckman & Hanson 2005).
Rhesus isoimmunisation and ABO incompatibility
Rhesus isoimmunisation (RhD incompatibility)
Readers should ensure they understand blood group inheritance before reading on. The fetus inherits a gene for the rhesus factor from each parent and as rhesus D is a dominant gene if one or two genes are inherited the baby will be rhesus positive (Rh+). Only if the baby inherits two recessive d genes will the blood group be rhesus negative (Rh−) (Fig. 14.1).
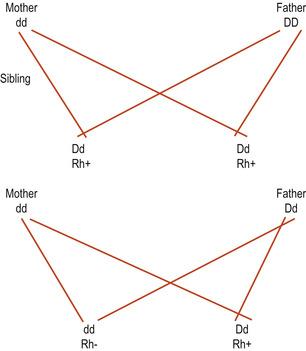 |
| Figure 14.1 Inheritance of the rhesus factor. (From Henderson C, Macdonald S 2004, with kind permission of Elsevier.) |
If the mother is Rh− and the fetus Rh +, haemolysis of fetal red cells may occur (Fig. 14.2). This rarely affects the first baby as there are no spontaneous anti-D antibodies present in a woman’s blood prior to her first pregnancy. This may happen if fetal red blood cells cross the placental barrier during that pregnancy or if she has been transfused accidentally with Rh+ blood.
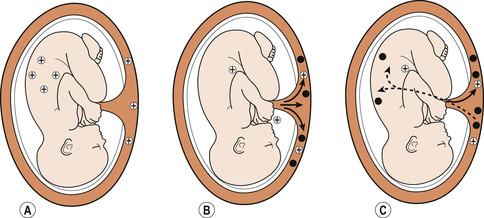 |
| Figure 14.2 Antibody formation. (A) Transfer of rhesus antigen (+) to the maternal circulation. (B) Antibody formation (•) in the rhesus negative mother. (C) Transfer of the rhesus antibody to the fetus. (From Henderson C, Macdonald S 2004, with kind permission of Elsevier.) |
During pregnancy and labour there is normally no mixing of maternal and fetal circulations. When the placenta separates, the chorionic villi tear and there is a risk of fetomaternal haemorrhage (FMH); usually between 0.5 ml and 5 ml of fetal blood enters the maternal circulation. If the fetus is Rh+ the production of antibodies will be stimulated and memory cells will mount a secondary response should the mother become pregnant with a second Rh+ baby. This process is called isoimmunisation.
Spontaneous or therapeutic abortion, amniocentesis, antepartum haemorrhage or external cephalic version may lead to an FMH and antibody formation. The problem arises in subsequent pregnancies because anti-D antibodies cross the placenta and haemolyse fetal red cells. Some protection occurs if the mother and fetus are ABO incompatible, as the naturally occurring anti-A or anti-B will destroy fetal red cells before the maternal immune system can respond to the rhesus factor.
Prevention of maternal isoimmunisation
In the past haemolytic disease of the newborn led to fetal or neonatal death from rhesus haemolytic disease but this is now largely preventable if three conditions are met:
1. Rhesus-positive blood should never be transfused if a woman’s blood group is unknown.
2. Unnecessary risk of FMH should be avoided, for example by placental localisation prior to amniocentesis. Abdominal palpation of women with an antepartum haemorrhage should be kept to a minimum.
3. If there has been a risk of FMH, anti-D immunoglobulin (rhesus D antibodies) must be administered to the mother within 72 h; this confers passive immunity for about 3 months. The antibodies will coat and destroy any fetal red cells in the maternal circulation.
The dose of anti-D immunoglobulin (Ig) is normally 250 IU (international units) before 20 weeks of pregnancy and 500 IU after 20 weeks. Occasionally the FMH is so large that one dose of anti-D is insufficient to prevent isoimmunisation. The number of fetal red cells in maternal blood is estimated by means of a Kleihauer test and, if a second dose is needed, the laboratory will inform the doctor.
Antenatal management: anti-D prophylaxis
Every pregnant woman has her blood tested for ABO and rhesus types early in pregnancy. Any rhesus-negative women will be screened for RhD antibodies. If the test is negative further screening will be carried out. It is now recommended that all non-sensitised women should receive anti-D prophylaxis of at least 500 IU, usually at 28 and 34 weeks of pregnancy. Because some fetomaternal bleeds are undetected in pregnancy, some authorities recommend the use of antepartum RhD Ig prophylaxis (NICE 2007).
Failure to adhere to protocols of anti-D Ig administration means there are still incidences of women becoming sensitised and their babies developing haemolytic disease of the newborn (Percival 2004).
• Not testing the size of an FMH to ensure an appropriate dose of anti-D Ig was given.
• Some women do not receive anti-D Ig, including those who attend accident and emergency departments with bleeding in early pregnancy.
• Not administering anti-D Ig for abdominal trauma in 80% of cases.
• Using an inadequate dose of anti-D Ig following antepartum haemorrhage.
• Not managing sensitising events occurring after 20 weeks gestation.
• Not understanding Kleihauer test results, with a negative result interpreted as a reason not to give anti-D Ig.
• Postnatal omissions resulting because of confusion with women who had recently received antenatal treatment with anti-D Ig.



