Coccidioides Lymphadenitis
Tariq Muzzafar, MBBS
Key Facts
Etiology/Pathogenesis
2 species: C. immitis prevalent in California, USA; C. posadasii in other regions
Clinical Issues
May be seen in nonendemic areas
Travel history should be sought
60% of infections are asymptomatic
Microscopic Pathology
Early acute phase: Neutrophils, histiocytes, eosinophils
Granulomatous phase
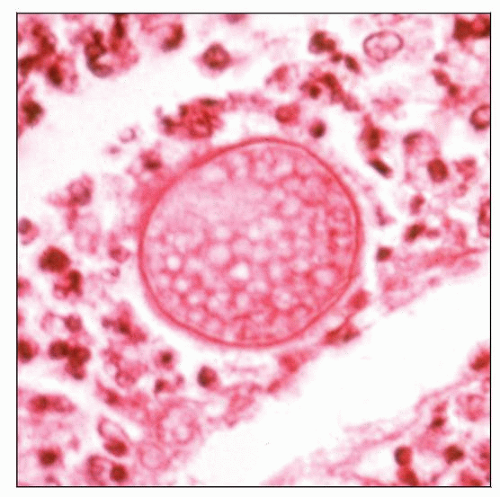
Round spherules (10-100 µm) in progressive developmental stages
Internal and external endospores (2-5 µm)
Ancillary Tests
Calcofluor white fluorescense sensitive
GMS and PAS stains highlight spherules
Recognition of endospores within spherules diagnostic
Culture yield variable and depends on site and phase of disease
Top Differential Diagnoses
Tuberculous lymphadenitis
Histoplasma lymphadenitis
Sarcoidosis
Kikuchi-Fujimoto lymphadenitis
Reporting Considerations
Classified as agents of potential bioterrorism in USA
Isolates should be reported to CDC within 7 days
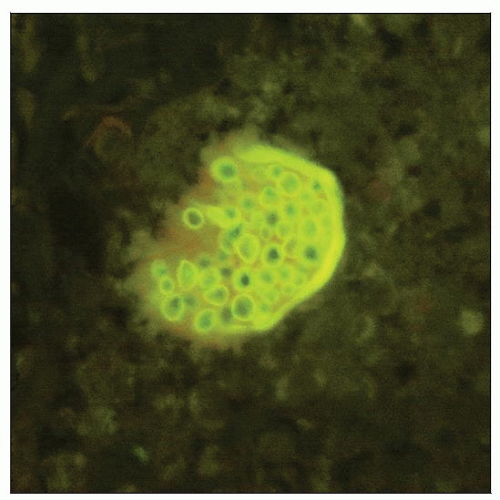 Spherule of C. immitis with endospores is demonstrated by Calcofluor white stain. (Courtesy B.J. Harrington, MD.) |
TERMINOLOGY
Definitions
Inflammation of lymph nodes due to Coccidioides immitis or Coccidioides posadasii
ETIOLOGY/PATHOGENESIS
Infectious Agents
2 species recognized
C. immitis, predominant in California, USA
C. posadasii, predominant in other regions
Epidemiology
Endemic in semi-arid to arid areas of
Southwest USA
Parts of South America
Organism
Grows in warm, sandy soil
Prevalent in areas having hot summers, mild winters, and < 20 inches of rainfall annually
Does not grow at altitudes above 3,700 feet
Occasional epidemics in past 30 years
Outbreaks can follow dust storms, earthquakes, and droughts
High-risk factors include
Occupational soil exposure
Agricultural workers
Military personnel
Archaeologists
Immunocompromised status as a result of
Organ transplant
Immunosuppressive agents
Acquired immune deficiency syndrome (AIDS)
Malignant diseases
Pregnancy
High risk of dissemination in
Filipinos
African-Americans
Subjects with blood group B
Incidence rising rapidly in USA due to
People settling in endemic areas
Growing immunocompromised population
New construction in uninhabited regions resulting in arthrospore dissemination
Increased awareness of disease entity
Cases seen in nonendemic areas due to increased travel
Travel history should be sought
High level of suspicion necessary
Incidence expected to rise in future
Pathogenesis
Coccidioides spp. are dimorphic
Mycelial phase
Grows in soil
Branching, septate hyphae
Can remain viable in dry desert soil for years
Multiplies after rainfall, forming arthroconidia
Arthroconidia
Separated by empty, thin-walled cells (disjunctors)
Dispersed into air and inhaled
Transform into multinucleated spherules within lung
Spherule phase
Spherules increase in size
Form thick outer wall
Divide to form numerous uninucleated endospores
Break open and release endospores, which form new spherules
Cycle continues
Spherules disseminate hematogenously to meninges, bones, skin, and soft tissue
Cell-mediated immunity crucial to limiting infection
Primary pulmonary infections asymptomatic in 60% of patients
Usual course of infection is healing without sequelae
Localized lesion (coccidioidoma) may persist
Reporting Considerations
C. immitis and C. posadasii classified as select agents of potential bioterrorism in USA
Laboratories must report findings to Centers for Disease Control (CDC) within 7 calendar days
Safety Considerations
Laboratory workers potentially at risk of accidental exposure
Biosafety level 2 practices and facilities recommended
Manipulation of clinical material conducted in class II biological safety cabinets
CLINICAL ISSUES
Presentation
General comments
Signs and symptoms
Wide spectrum
Similar to community-acquired pneumonia
60% of patients are asymptomatic
Most common infections self-limited and misdiagnosed
Disseminated disease in < 5% of symptomatic patients
Acute pneumonia
Presents 1-3 weeks after inhalation of arthroconidia
Profound fatigue
Lobar infiltrates and lymphadenopathy in patient who has traveled to endemic area are suggestive
Pleural effusion in 5-10% cases
Erythema multiforme, erythema nodosum, toxic erythema (immune mediated)
Diffuse pneumonia
Due to
Inhalation of large number of arthrospores
Hematogenous spread
Immunocompromised status
Severe illness, high fever, dyspnea, hypoxemia
Can progress to acute respiratory distress syndrome
Chronic progressive pneumonia
Persistent illness lasting > 3 months in small percentage of patients
Persistent coughing, sputum production, hemoptysis
Weight loss
Serologic testing positive
Pulmonary nodules and cavities
Can be initial presentation of primary infection
Can occur in immunocompetent hosts after infiltrate resolves
1-2 cm nodule or cavity
Cavity may wax and wane
Usually do not cause symptoms
Cough, chest pain, and hemoptysis may occur
Rupture of cavity near pleural surface may lead to hydropneumothorax
Extrapulmonary noncentral nervous system disease
Occurs in < 5% of immunocompetent patients and in high-risk groups
Skin, lymph nodes, bones, and joints involved
Diagnosed several months after onset of pulmonary symptoms
Surgical excision may be necessary
Central nervous system disease
Granulomatous meningitis or coccidioidoma
Headache, mental status changes, neurologic deficits
Serologic studies essential for diagnosis
Laboratory Tests
Peripheral blood
Elevated erythrocyte sedimentation rate
Eosinophilia
Pleural fluid
Usually exudative
May show eosinophilia
Cerebrospinal fluid (CSF)
Increased white blood cells, predominantly lymphocytes
Increased protein, decreased glucose
Light microscopy
Round spherules (10-100 µm) in progressive developmental stages
Internal and external endospores (2-5 µm)
Recognition of endospores within spherules considered diagnostic
Few spherules without internal structures considered presumptive evidence
May be seen in giant cells, microabscesses, and in acute presentations
Less likely to be found in caseous, calcified, or liquefactive foci
Rarely seen in CSF in meningitis
Immature spherules in contact may simulate Blastomyces
Endospores without spherules (especially in CSF) may simulate Histoplasma, Cryptococcus, Candida
Mycelia may be identified in
Boundaries of old cavitary lung lesions
Skin lesions
Ventricular fluid in CNS infection
Mycelia without spherules not diagnostic
Culture isolates show slender, hyaline, and septate hyphae
Arthroconidia
Unicellular, barrel-shaped (3-4 x 3-6 µm)
Arise from side branches
Alternate with thin-walled, empty disjunctor cells
Released at maturity
Calcofluor white (CFW) fluorescence
Binds chitin and cellulose in fungal cell wall
Sensitive but may stain plant material
Rapid results
May be used on tissue, body fluids, respiratory secretions
KOH wet mount
Not as sensitive as CFW
Grocott methenamine silver
Most sensitive histopathologic stain
However, may obscure endospores within spherules
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree