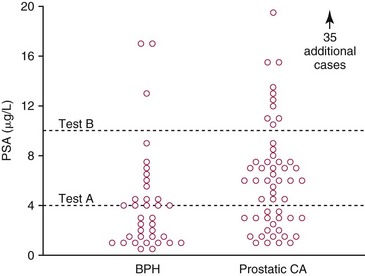
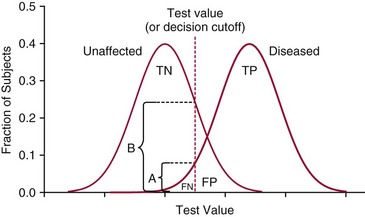
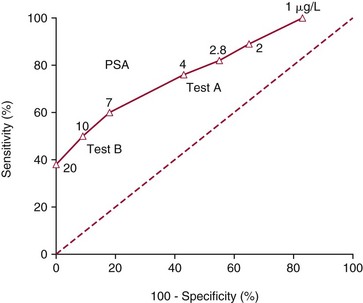
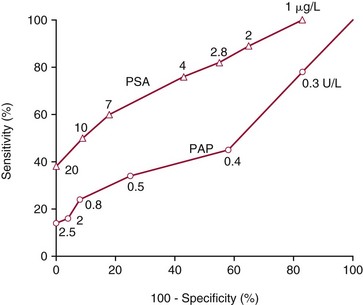
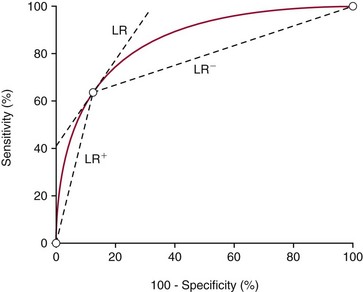
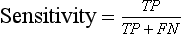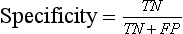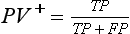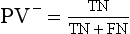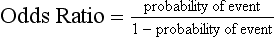Chapter 3 A vast majority of medical decisions rely on laboratory testing. Clinicians often ask which test or sequence of tests (1) provides the best information in a specific setting, (2) is the most cost-effective, and (3) offers the most efficient route to diagnosis or considered medical action. In addition, it is often asked, “How does one combine a testing result or testing information with previously obtained information?” In addressing these questions, this chapter focuses on how to use the diagnostic information obtained from a test or a group of tests, and how to compare test results with those of other tests. Designing studies to assess diagnostic accuracy is addressed in Chapter 4, Evidence-Based Laboratory Medicine. The analytical performance of the methods used for many clinical tests has improved dramatically. However, a test that has high analytical accuracy and precision may provide less useful clinical information than a test that performs worse analytically. For example, a test for free ionized calcium is often more accurate and precise than one for parathyroid hormone (PTH), yet knowledge of ionized calcium is of less value in the assessment of hyperparathyroidism. Pertinent questions include: (1) How does one evaluate the information content of a test? And (2) What procedure should one use to decide among different tests based on their disease discrimination ability? This chapter discusses these and other nonanalytical aspects of test performance that affect a test’s overall medical usefulness. Although the techniques described in this chapter have been recommended to clinicians for nearly two decades, few physicians avail themselves of their use. Laboratorians need to take a more active role in promoting these techniques.5 The sensitivity of a test reflects the fraction of those with a specified disease that the test correctly predicts. The specificity is the fraction of those without the disease that the test correctly predicts. Table 3-1 shows the classification of unaffected and diseased individuals by test result. True positives (TP) are those diseased individuals who are correctly classified by the test. False positives (FP) are nondiseased individuals misclassified by the test. False negatives (FN) are those diseased patients misclassified by the test. True negatives (TN) are nondiseased patients correctly classified by the test. TABLE 3-1 Classifications of a Test Result Applied to Unaffected and Diseased Populations An example of a dichotomous test is the human immunodeficiency virus (HIV) screening test. This test detects HIV antibodies, producing results that may be nonreactive (negative) or reactive (positive). False positives occur owing to technical errors such as mislabeling or contamination and the presence of cross-reacting antibodies found in individuals such as multiparous women and multiply transfused patients.28 False negatives occur because of technical errors such as mispipetting and sampling determinants such as testing in early infection (3 to 4 weeks) prior to antibody production. Reported sensitivities and specificities for the HIV screening test vary widely,16 but reasonable estimates are 96% and 99.8%, respectively. Thus, 4 of 100 HIV-infected subjects will test negative. Only 2 of 1000 noninfected subjects will test positive. The clinical usefulness of an HIV test result from an unknown subject will be explained later in the “Probabilistic Reasoning” section. Figure 3-1 is a dot plot of the performance of a continuous assay for prostatic-specific antigen (PSA) in patients with benign prostatic hyperplasia (BPH) and in those with established carcinoma of the prostate (stages A through D).8 Often continuous tests are used in a dichotomous fashion by choosing one or more decision cutoffs. Note the two dashed lines crossing the graphs that represent two diagnostic cutoffs. Both tests A and B are PSA tests, but they have different decision cutoffs, namely, 4 µg/L and 10 µg/L. When test A is compared with test B, the decision cutoff of 4 µg/L for test A produces increased sensitivity but at the cost of a decrease in specificity. Thus increased true-positive detection has been traded for an increase in the number of false-positive results. This tradeoff occurs in every test performed in medicine. Not only does it affect the interpretation of quantitative laboratory results, it also affects the opinions of surgical pathologists and radiologists and of the care provider who performs a physical examination. Figure 3-2 illustrates a hypothetical test that shows higher results in patients who have a disease compared with those who are unaffected. As the decision cutoff is increased, FP decrease and FN increase. At extremely low and extremely high cutoffs, sensitivity and specificity are 100%. The dot plot (see Figure 3-1) displays quantitative performance in a limited fashion. For example, one cannot easily estimate sensitivity and specificity for various decision cutoffs using the dot plot. A graphical technique for displaying the same information, called a receiver operating characteristic (ROC) curve, began to be used during World War II to examine the sensitivity and specificity associated with radar detection of enemy aircraft. An ROC curve is generated by plotting sensitivity (y-axis) versus 1 − specificity (x-axis).10a Figure 3-3 shows the ROC curve for the data in Figure 3-1. The x-axis plots the fraction of nondiseased patients who were erroneously categorized as positive for a specific decision threshold. This “false-positive rate” is mathematically the same as 1 − specificity. The y-axis plots the “true-positive rate” (the sensitivity). A “hidden” third axis is contained within the curve itself: the curve is drawn through points that represent different decision cutoff values. Those decision cutoffs are listed as labels on the curve.18 The entire curve is a graphical display of the performance of the test. Tests A and B from Figure 3-3 are displayed as two decision points on the ROC curve. The dotted line extending from the lower left to the upper right represents a test with no discrimination and is designated the random guess line. A curve that is “above” the diagonal line describes performance that is better than random guessing. A curve that extends from the lower left to the upper left and then to the upper right is a perfect test. The area under the curve describes the test’s overall performance, although usually one is interested only in its performance in a specific region of the curve.1 One strength of the ROC graph lies in its provision of a meaningful comparison of the diagnostic performance of different tests. In the medical literature, the use of 2 × 2 tables to present the sensitivity and specificity of a test has led to the common logical misconception that a quantitative test has a single sensitivity and specificity. When the initial publication of an assay recommends a cutoff for analysis purposes, the assay is often categorized as sensitive or specific based on this cutoff. Yet, as seen in the ROC curve, every assay can be as sensitive as desired at some cutoff, and as specific as desired at another. When two procedures are compared, confusion is avoided by using ROC curves instead of accepting statements such as, “Test A is more sensitive, but test B is more specific.” For example, the usefulness of the prostatic acid phosphatase assay had been compared for years with that of the PSA assay for diagnostic and follow-up purposes. Various claims were made regarding the relative sensitivity and specificity of the two assays.12,35 Figure 3-4 compares the performance of an acid phosphatase assay with that of the PSA assay for discrimination between BPH and prostatic carcinoma in the same cohort of patients. Although each test has been claimed to be “more sensitive but less specific” than the other by various authors, it is clear from the ROC curves that the authors were choosing different points on the two curves. No matter what level of sensitivity is chosen, the PSA assay offers greater specificity than the acid phosphatase assay at the same level of sensitivity. This does not mean that one should conclude that the PSA assay is always superior. It does indicate that for the cohort of patients used to compare the assays, the PSA assay offers superior performance compared with the prostatic acid phosphatase assay. However, the acid phosphatase assay may provide superior diagnostic information to that provided by the PSA assay in subpopulations of the cohort. The area under the ROC curve is a relative measure of a test’s performance. A Wilcoxon statistic (or equivalently, the Mann-Whitney U-test) statistically determines which ROC curve has more area under it. These methods are particularly helpful when the curves do not intersect. When the ROC curves of two laboratory tests assessing for the same disease intersect, the tests may exhibit different diagnostic performances, even though the areas under the curve are identical. Test performance depends on the region of the curve (i.e., high sensitivity vs. high specificity) chosen. Details on how individual points on two curves can be compared statistically have been provided elsewhere.1 Interpretation of almost all laboratory test results is affected by the probability of the disorder prior to testing. For example, an elevated PSA concentration in a 35-year-old is not interpreted in the same way as in a 70-year-old because the rate of occurrence of prostatic cancer in 35-year-olds is much lower than that in older men.29 Interpretation must be tempered by knowledge of the prevalence of the disease. Prevalence is defined as the frequency of disease in the population examined.44 For example, with step sectioning of prostate tissue from a random sample of men older than 50 years of age, at least a 25% probability of histologic carcinoma is expected (most of the carcinomas identified will never become clinically important, but they are carcinomas nevertheless).17,34 Several useful techniques have been applied to combine the prevalence with information previously obtained in the results of testing. Predictive values are a function of sensitivity, specificity, and prevalence. It is regrettable that clinicians often confuse sensitivity with PV+. For example, suppose that 1,000,000 U.S. residents were randomly chosen and tested for HIV infection using the HIV screening test. The Centers for Disease Control and Prevention estimates that the prevalence of HIV infection in the United States is 330.4 per 100,000 population.7 On the basis of this prevalence, about 3304 infected individuals would be expected in a population of 1 million. Because the sensitivity of the HIV test is 96%, about 3172 infected individuals would have a positive test result (i.e., TP = 3172). Similarly, because the specificity of the HIV test is 99.8%, about 2 false positives per 1000 subjects would be expected. Thus about 1993 individuals would have false-positive results (i.e., FP = 1993). Therefore, the PV+ is 3172/(3172 + 1993), or 61%. Thus an individual with a positive test result has a moderate chance of having a false-positive result. Additional testing is necessary to separate TP individuals from FP individuals. Most laboratories automatically test all specimens having a positive HIV screening result with a confirmatory test such as the HIV Western blot (see Chapter 12). The likelihood ratio (LR) is the probability of occurrence of a specific test value given that the disease is present divided by the probability of the same test value if the disease was absent. Many sources (e.g., Henry20) indicate that the slope of the ROC curve is equal to the LR for a given test value. Assertions such as these oversimplify the concept of LR. Choi10 describes three different slopes of the ROC curve, which represent LR in different settings (as illustrated in Figure 3-5): Figure 3-5 Receiver operating characteristic curve illustrating the slopes that define the likelihood ratio (LR) for a continuous test at a specific test result (the gray point), and the positive likelihood ratio (LR+) and the negative likelihood ratio (LR−) of a dichotomous test.10 1. The tangent slope, which is equal to the LR of a continuous test at a given test value. 2. The slope from the origin to a test value equal to a decision cutoff, the LR+ for a positive result of a dichotomous test; this slope has a companion slope (which is the slope from the cutoff value to the upper right hand corner of the ROC plot), which represents the LR− for a negative result of a dichotomous test. 3. A slope between any two test values (not illustrated in Figure 3-5), which is termed the interval LR and represents the LR of a result that lies between the values; the interval LR is useful for continuous tests that have results grouped into intervals. For quantitative tests, the LR is the tangent slope of the ROC curve, which equals the ratio of the heights A and B of the two curves at the test value in Figure 3-2. Note that the areas under each curve in Figure 3-2 are the same. The likelihood ratio does not take disease prevalence or any other prior information into account. To arrive at a final probability, one must adjust for the best estimate of the probability of disease before obtaining the test result.
Clinical Utility of Laboratory Tests
Diagnostic Accuracy of Tests
Sensitivity and Specificity
No. of Patients With Positive Test Result
No. of Patients With Negative Test Result
No. of patients with disease
TP
FN
No. of patients without disease
FP
TN
Receiver Operating Characteristic Curves48
Probabilistic Reasoning
Prevalence
Predictive Values
Likelihood Ratio
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


Basicmedical Key
Fastest Basicmedical Insight Engine