Chapter 9 Michael A. Pesce; Alex J. Rai; Jorge L. Sepulveda; Serge Cremers Questions ■ Potassium: 3.5 to 5.0 mEq/L ■ pH: 7.38 to 7.44 ■ Naurine: 10 to 26 mEq/L A. Glucose = 100 mg/dL; K = 3.2 mEq/L; pH = 7.3; Naurine = 30 meq/L. B. Glucose = 500 mg/dL; K = 6.9 mEq/L; pH = 7.1; Naurine = 30 meq/L. C. Glucose = 100 mg/dL; K = 2.9 mEq/L; pH = 7.1; Naurine = 55 meq/L. D. Glucose = 180 mg/dL; K = 3.2 mEq/L; pH = 7.3; Naurine = 55 meq/L. E. Glucose = 40 mg/dL; K = 4.0 mEq/L; pH = 7.3; Naurine = 30 mEq/L. 2. An 18-month-old child with a history of respiratory infections had a sweat chloride result of 52 mmol/L. According to the Cystic Fibrosis Foundation, a sweat chloride level of 60 mmol/L or higher is suggestive of cystic fibrosis; a chloride level between 40 and 59 mmol/L is an intermediate level; and a chloride level of 39 mmol/L or less is considered normal. Based on this test result in this patient, which one of the following is the most appropriate next step in the workup? A. Diagnose cystic fibrosis based on this chloride level and clinical symptoms. B. Measure immunoreactive trypsinogen levels in the blood to confirm the diagnosis of cystic fibrosis. C. Diagnose this individual as a cystic fibrosis carrier with a mutated cystic fibrosis transmembrane conductance regulator (CFTR) gene. D. Collect another sweat sample and repeat the chloride measurement. E. Measure serum sodium and chloride levels to confirm the diagnosis of cystic fibrosis. 3. Which one of the following is the most common quantitative method used to measure sweat chloride concentrations in patients suspected of having cystic fibrosis? A. Ion-selective electrode (ISE) with a potentiometric end point. B. Mercuric ferric thiocyanate-colorimetric method. C. Enzymatic measurement. D. Coulometric titration procedure. E. Isotopic dilution mass spectrometry method. 4. Which one of the following tests is used to diagnose cystic fibrosis? A. Measurement of sweat osmolality. B. Measurement of immunoreactive trypsinogen. C. Measurement of sweat chloride. D. Measurement of sweat conductivity. E. Measurement of serum chloride. 5. Arterial blood gas measurements should be performed as soon as possible after the blood sample is obtained from the patient. Which one of the following will occur as a result of glycolysis if the blood is kept in the syringe at room temperature for 1 hour? A. Increased pH, Po2, and Pco2. B. Decreased pH and increased Po2 and Pco2. C. Decreased pH and Pco2 and increased Po2. D. Decreased pH and Po2 and increased Pco2. E. Decreased pH, Po2, and Pco2. 6. An arterial blood gas sample, collected in a heparinized syringe from a patient who was not receiving oxygen therapy, was sent to the laboratory on ice but was exposed to room air for approximately 30 minutes. Which one of the following sets of changes in the blood gas measurements will occur? A. Increased Po2, pH, and Pco2. B. Decreased Po2, pH, and Pco2. C. Increased Po2 and pH and decreased Pco2. D. Unchanged Po2 and decreased Pco2 and pH. E. Decreased Po2 and pH and increased Pco2. 7. Which one of the following techniques is used to correct for interferences in the automated Jaffe reaction? A. Online dialysis to remove proteins. B. Fluorescence measurement of the color produced. C. Spectrophotometric measurement of the color produced at two different wavelengths. D. Spectrophotometric measurement of the color produced after the reaction has gone to completion. E. Kinetic assay, where the reaction rate is measured between 20 and 60 seconds. 8. Which one of the following combinations of the concentration of urea (in both mg/dL and mmol/L) is equivalent to a serum blood urea nitrogen (BUN) concentration of 15 mg/dL? ■ Atomic weights: N = 14, C = 12, O = 16, H = 1. B. 32 mg/dL and 5.3 mmol/L. C. 7 mg/dL and 5.3 mmol/L. D. 7 mg/dL and 11.4 mmol/L. E. 64 mg/dL and 15 mmol/L. 9. A physician orders multiple tests for her patient in the intensive care unit. Postprandial samples are collected in a blood gas syringe and in a serum separator tube (SST). Electrolytes are measured on both sample types and a discrepancy is noted in the sodium concentration (i.e., [Na+]) when comparing the results obtained with the blood gas analyzer (syringe) and the automated laboratory analyzer (SST). Which one of the following is the most likely explanation for this discrepancy? A. Higher imprecision using the blood gas analyzer. B. Wider dynamic range with the automated laboratory analyzer. C. Lower coefficient of variation with the automated laboratory analyzer. D. Different methods are used for this analysis on these two instruments. E. Greater accuracy using the automated laboratory analyzer. 10a. Which one of the following sets of laboratory results on a serum sample is most consistent with primary hypoparathyroidism? A. Decreased intact parathyroid hormone (PTH), increased calcium. B. Increased intact PTH, increased calcium. C. Decreased intact PTH, decreased calcium. D. Increased intact PTH, decreased calcium. E. Increased intact PTH, calcium within the normal range. 10b. Which one of the following sets of laboratory results on a serum sample is most consistent with the presence of malignancy? A. Decreased intact PTH, increased calcium. B. Increased intact PTH, increased calcium. C. Decreased intact PTH, decreased calcium. D. Increased intact PTH, decreased calcium. E. Increased intact PTH, calcium within the normal range. 10c. Which one of the following sets of laboratory results on a serum sample is most consistent with primary hyperparathyroidism? A. Decreased intact PTH, increased calcium. B. Increased intact PTH, increased calcium. C. Decreased intact PTH, decreased calcium. D. Increased intact PTH, decreased calcium. E. Decreased intact PTH, calcium within the normal range. 10d. Which one of the following sets of laboratory results on a serum sample is most consistent with secondary hyperparathyroidism due to renal failure? A. Decreased intact PTH, increased calcium. B. Increased intact PTH, increased calcium. C. Decreased intact PTH, decreased calcium. D. Increased intact PTH, decreased calcium. E. Decreased intact PTH, calcium within the normal range. 11. The fractional hemoglobin saturation (i.e., Fo2Hb) refers to the percentage of total hemoglobin that is saturated with oxygen. If the oxygen saturation (So2) is known, which one of the following choices identifies the two additional parameters necessary for calculating the Fo2Hb? A. Carboxyhemoglobin and deoxyhemoglobin. B. Deoxyhemoglobin and methemoglobin. C. Sickle hemoglobin and methemoglobin. D. Sickle hemoglobin and deoxyhemoglobin. E. Carboxyhemoglobin and methemoglobin. 12. Which one of the following patterns is most likely to be observed in patients with primary hyperparathyroidism? A. Elevated serum and urine levels of calcium and inorganic phosphate. B. Elevated levels of serum calcium and inorganic phosphate, elevated levels of urine calcium, and low levels of urine inorganic phosphate. C. Elevated levels of serum and urine calcium and low levels of serum and urine inorganic phosphate. D. Elevated levels of serum calcium, low levels of serum inorganic phosphate, low levels of urine calcium, and elevated levels of urine inorganic phosphate. E. Elevated levels of serum and urine calcium, low levels of serum inorganic phosphate, and elevated levels of urine inorganic phosphate. 13. In assessing hyponatremia, which one of the following analytes is least important? B. Plasma sodium. C. Urine sodium. D. Plasma calcium. E. Plasma glucose. 14. Which one of the following settings will lead to a falsely decreased plasma sodium concentration when measured by an indirect ISE method? A. Elevated circulating chloride and glucose levels. B. Elevated circulating protein and lipid levels. C. Decreased circulating protein and lipid levels. D. Decreased circulating protein and glucose levels. E. Decreased circulating protein and chloride levels. 15. Which one of the following is the most likely cause of a low BUN/creatinine ratio? B. A high-protein diet. C. Acute tubular necrosis. D. Acute glomerular injury. E. Severe liver disease. 16. Which one of the following best describes the use of the Modification of Diet in Renal Disease (MDRD) equation for estimating the glomerular filtration rate (GFR)? B. The calculation requires the serum creatinine and BUN levels along with patient age, gender, and race. C. The calculation requires the serum creatinine and cystatin C levels along with patient age, gender, and race. D. The calculation requires the serum and urine creatinine levels along with the volume of urine. E. The calculation uses the serum creatinine level along with patient age, gender, and race. 17. A patient has the laboratory results shown in Table 9-1. Which one of the following conditions is the most likely underlying cause of these results? A. Metabolic acidosis with an elevated anion gap. B. Metabolic acidosis with a normal anion gap. C. Respiratory acidosis with an elevated anion gap. D. Renal tubular acidosis (RTA). E. Respiratory acidosis with a normal anion gap. 18. Which one of the following would most likely be seen in a patient with severe diarrhea? A. Metabolic alkalosis with an elevated anion gap. B. Respiratory acidosis with an elevated anion gap. C. Metabolic acidosis with a normal anion gap. D. Metabolic acidosis with an elevated anion gap. E. RTA type 2 with an elevated anion gap. 19. A patient has the laboratory results shown in Table 9-2. Which one of the following is the most likely cause of these results? B. Respiratory acidosis. C. Metabolic alkalosis. D. Diabetic ketoacidosis (DKA). E. Respiratory alkalosis. 20. Blood gas analysis was performed on a patient sample and the following results were obtained: pH = 7.40 (reference range, 7.36 to 7.41), Pco2 = 55 mm Hg (reference range, 32 to 45 mm Hg), and HCO3− = 46 mmol/L (reference range, 22 to 29 mmol/L). Which one of the following conditions is the most likely underlying cause of these results? A. Metabolic alkalosis with respiratory compensation. B. Metabolic alkalosis. C. Metabolic acidosis. D. Respiratory acidosis. E. Respiratory alkalosis with metabolic compensation. 21. Which one of the following best describes the significance of microalbuminuria? A. Predicts glomerulonephritis. B. Contributes to multiple myeloma staging. C. Identifies the presence of end-stage renal disease. D. Predicts kidney transplant rejection. E. Predicts diabetic nephropathy. 22. Which one of the following answers best describes the most significant laboratory result(s) for identifying nephrotic syndrome? A. Increased serum BUN and creatinine concentrations. B. Increased serum cystatin C concentration. C. Increased serum cholesterol concentration. D. Microalbuminuria. E. Proteinuria of greater than 3.0 g/day. A 58-year-old man who underwent colon resection was treated with an aminoglycoside for a surgical infection. A day and a half after beginning this therapy, his creatinine jumped from 0.8 mmol/L to 3.8 mmol/L (reference range, 0.6 to 1.2 mmol/L) with a BUN of 60 mg/dL (reference range, 7 to 20 mg/dL). Use this scenario to answer the following two questions. 23a. Which one of the following would be the next most helpful diagnostic test? B. Serum and urine osmolality. C. Serum potassium. D. Plasma aminoglycoside levels. E. Renal biopsy. 23b. The patient’s renal failure was determined to be of tubular etiology based on the diagnostic test you recommended above. Which one of the following is the most likely serum calcium, ionized calcium, and phosphate, respectively, that would be seen in this patient? Reference ranges are as follows: ■ Ionized calcium (4.5 to 5.3 mg/dL) ■ Phosphate (2.5 to 4.3 mg/dL) A. 6 g/dL, 0.99 g/dL, 4 g/dL. B. 6 g/dL, 0.89 g/dL, 6 g/dL. C. 9 g/dL, 0.89 g/dL, 6 g/dL. D. 9 g/dL, 0.99 g/dL, 4 g/dL. E. 6 g/dL, 0.99 g/dL, 2 g/dL. 24. A patient has the laboratory results shown in Table 9-3. Which one of the following conditions is the most likely underlying cause of these results? A. Metabolic acidosis with a normal anion gap. B. Metabolic acidosis with an elevated anion gap. C. Respiratory acidosis with an elevated anion gap. D. RTA with an elevated anion gap. E. Respiratory acidosis with a normal anion gap. 25. A patient has the laboratory results shown in Table 9-4. Which one of the following conditions is the most likely underlying cause of these results? B. Respiratory acidosis. C. Metabolic alkalosis. D. Respiratory alkalosis. E. DKA. 26. Which one of the following mechanisms physiologically compensates for a respiratory alkalosis? B. Renal regulation of HCO3−. C. Hyperventilation. D. Renal excretion of H+. E. Renal excretion of organic acids. 27. Which one of the following serum patterns is most likely to be observed in children with rickets? A. Low levels of calcium, 25-hydroxyvitamin D, inorganic phosphate, and PTH. B. Low levels of calcium, 25-hydroxyvitamin D, and inorganic phosphate, and an elevated PTH level. C. Low levels of calcium and 25-hydroxyvitamin D, and elevated levels of inorganic phosphate and PTH. D. Low levels of calcium, 25-hydroxyvitamin D, and PTH, and an elevated inorganic phosphate level. E. Elevated levels of calcium and PTH and low levels of 25-hydroxyvitamin D and inorganic phosphate. 28. So2 refers to the percent of functional hemoglobin (Hb) that is saturated with oxygen. Which one of the following pairs identifies the two forms of Hb that are necessary for this calculation? A. Oxyhemoglobin and carboxyhemoglobin. B. Oxyhemoglobin and deoxyhemoglobin. C. Oxyhemoglobin and methemoglobin. D. Deoxyhemoglobin and carboxyhemoglobin. E. Deoxyhemoglobin and methemoglobin. 29. A 35-year-old asymptomatic woman has the laboratory results shown in Table 9-5. Which one of the following is the most likely explanation for these results? A. Anticoagulant contamination. B. Chronic kidney failure. C. Renal tubular acidosis. D. Primary hyperaldosteronism. E. Congenital adrenal hyperplasia. A 38-year-old woman presented for elective cholecystectomy with the preoperative laboratory values shown in Table 9-6. Use this scenario to answer the following three questions. 30a. A previous electrolyte profile performed 2 months ago showed similar results, except for sodium of 145 mmol/L, creatinine of 1.1 mg/dL, and BUN of 23 mg/dL. The patient reported that for the last 5 months she had increased dyspnea on exertion and ingested 5 to 6 cups of water a night, except the previous night, when she was fasting for the next day’s operation. Which one of the following is the most likely diagnosis? B. Conn syndrome. C. Syndrome of inappropriate antidiuretic hormone secretion. D. Diabetes insipidus. E. Diabetes mellitus. 30b. On further testing, the chest radiograph revealed bilateral hilar adenopathy and fine reticular opacities; blood testing showed serum calcium 13.5 mg/dL (reference range, 8.7 to 10.2 mg/dL), phosphorus 5.7 mg/dL (reference range, 2.5 to 4.8 mg/dL), PTH 15 pg/mL (reference range, 50 to 330 pg/mL), 25-hydroxyvitamin D 25 ng/mL (reference range, 30 to 74 ng/mL), antidiuretic hormone (ADH) 12 pg/mL (reference range, 4 to 12 pg/mL) with serum osmolality 300 mOsm/kg (reference range, 285 to 295 mOsm/kg), and cortisol 6 mg/dL (reference range, 5 to 23 mg/dL). Urinalysis was unremarkable except for urine osmolality 80 mOsm/kg (> 850 mOsm/kg with fluid restriction). Which one of the following clinical conditions best explains these results? A. Ectopic adrenocorticotropic hormone (ACTH) production. B. Lung cancer–producing ADH. C. Nephrogenic diabetes insipidus. D. Central diabetes insipidus. E. Diabetic nephropathy. 30c. Clinical evaluation and elevated plasma angiotensin-converting enzyme levels confirmed a past history of pulmonary sarcoidosis. Which one of the following laboratory results is most consistent with the patient’s diagnosis? A. Unchanged cortisol after ACTH administration B. Increased urine osmolality after water deprivation and ADH administration. C. Elevated 1,25-dihydroxyvitamin D. D. Elevated PTH-related peptide (PTHrP). E. Elevated C-peptide after a standard meal. 31. A 65-year-old man with a history of chronic myeloid leukemia presented with a white cell count of 185 × 109/L (reference range, 3.04 to 9.06 × 109/L), with 54% blasts, 26% myeloid precursors, 15% neutrophils, 4% basophils, 1% eosinophils, platelet count 256 × 109/L (reference range, 165 to 415 × 109/L), hemoglobin 11.5 g/dL (reference range, 13.3 to 16.2 g/dL), and normal basic metabolic panel except for a glucose of 60 g/dL (reference range, 65 to 95 g/dL) and potassium of 2.5 mmol/L (reference range, 3.5 to 5.5 mmol/L). Vital signs, neurological examination, and electrocardiogram were normal. Which one of the following most likely accounts for these findings? A. Rhabdomyolysis secondary to blood hyperviscosity. B. Adrenal insufficiency due to leukemic infiltration. C. Acute tubular necrosis of the kidney. D. Pseudohypokalemia due to leukocytosis. E. Lactic acidosis due to leukemic anaerobic metabolism. 32. A diabetic 56-year-old man with a history of type 2 diabetes presents to the emergency department with weakness, nausea, altered mental status, and the laboratory results shown in Table 9-7. Which one of the following statements most likely corresponds to the clinical situation? A. The normal BUN/creatinine ratio is inconsistent with dehydration. B. The hyponatremia is probably due to increased extracellular water content. C. The hyperkalemia indicates excess total body potassium. D. There is significant ketoacidosis due to insulin deficiency. E. Effective plasma osmolality is consistent with a hyperosmolar hyperglycemic state. 33. A 13-month-old girl with consanguineous parents presented with polydipsia, diuresis, vomiting, and lethargy and showed signs of dehydration, deep sighing respiration, and smelled of ketones. Her lab results are shown in Table 9-8. Her urine was strongly positive for ketones and urine culture grew Escherichia coli. Levels of hemoglobin A1c were 5.3% (reference range, normal < 5.7%), C-peptide was within normal range, and autoantibodies against glutamic acid decarboxylase and islet cells were not present. Urine organic acid analysis revealed elevated levels of methylmalonic acid. Plasma levels of vitamin B12, folate, and homocysteine were normal. Plasma acylcarnitine analysis showed elevation in C3 carnitine esters. Plasma lactate was elevated (36 mg/dL; reference range, 5 to 15 mg/dL) with normal pyruvate. The patient gradually improved after treatment of the urinary tract infection, initiation of a low-protein diet, cyanocobalamin, and l-carnitine supplementation. Which one of the following is the best explanation for this patient’s results? A. Type 1 diabetes complicated by urinary tract infection. B. Fatty acid oxidation defect. C. Pyruvate dehydrogenase deficiency. D. Malnutrition with chronic vitamin B12 deficiency. E. Adenosylcobalamin synthesis defect. 34. Which one of the following statements is true regarding physiological preanalytical effects on electrolyte levels? A. A standard meal will decrease plasma phosphate levels within 2 hours. B. A shift from supine to upright position causes increases in plasma calcium. C. Acute ethanol intoxication is frequently associated with metabolic alkalosis. D. Vigorous exercise often leads to lower potassium levels. E. Stress (e.g., during acute myocardial infarction) often causes hyperkalemia. 35. A 25-year-old asymptomatic woman was hospitalized for persistent plasma hyperkalemia of approximately 7.0 mmol/L (reference range, 3.5 to 5.0 mmol/L), first identified during routine blood work. Physical examination and electrocardiogram were normal. Examination of the plasma supernatant showed no evidence of hemolysis. On a subsequent phlebotomy, both plasma and serum samples were measured, which showed potassium levels of 5.6 and 4.3 mmol/L, respectively. When a blood sample was collected and immediately centrifuged, the potassium levels were 4.3 mmol/L, but incubation at room temperature for 6 hours resulted in potassium levels of 7.5 mmol/L. Which one of the following is the best explanation for these results? A. Ethanol contamination during phlebotomy. B. Prolonged tourniquet time with fist clenching. C. Thrombocytosis. D. Leukocytosis. E. Familial pseudohyperkalemia. A 26-month-old girl with mild developmental delay and at the 5th percentile for height has the laboratory results shown in Table 9-9. Urinalysis showed pH 5.0 and was positive for glucose without other abnormalities. Use this scenario to answer the following two questions. 36a. Which one of the following best explains the patient’s presentation? B. Type 1 (distal) RTA. C. Type 2 (proximal) RTA. D. Hyporeninemic hypoaldosteronism. E. DKA. 36b. Which one of the following test results would most likely be found on confirmatory testing? A. High urinary levels of methylmalonic acid by gas chromatography–mass spectrometry. B. Increased urinary pH after NH4Cl load. C. Elevated fractionary urinary excretion of HCO3 after bicarbonate load. D. Decreased plasma aldosterone/renin ratio. E. High plasma levels of β-hydroxybutyrate. 37. A 19-year-old man presented with low-grade fever, coughing, sore throat, and conjunctivitis. Blood counts and a basic metabolic panel were within the reference ranges except for a total CO2 of 15 mmol/L (reference range, 22 to 30 mmol/L). The physician was concerned about an acid-base disturbance, so an arterial blood gas analysis was performed (Table 9-10). Which one of the following best explains these findings? B. Respiratory alkalosis. C. Metabolic acidosis with respiratory compensation. D. Spuriously low venous bicarbonate due to hypotension. E. Spurious loss of CO2 in the venous sample. 38. A full-term boy was born after an uneventful pregnancy from consanguineous parents with 3.4-kg birth weight. Apgar scores (9 and 9) were normal. The next day after delivery, the baby presented with increased lethargy, jitteriness, and refusal to feed. During the admission, the baby developed respiratory failure and status epilepticus. Laboratory results are shown in Table 9-11. Plasma amino acid levels were normal except for plasma glutamine of 2870 nmol/mL (reference range, < 1060 nmol/mL), alanine of 3800 nmol/mL (reference range, < 820 nmol/mL), and undetectable citrulline. Orotic acid and reducing sugars were absent in the urine. Acylcarnitine profile was normal. Which one of the following enzymatic defects is most consistent with these findings? A. Carbamoyl phosphate synthase (CPS). B. Argininosuccinate lyase (ASL). C. Carnitine palmitoyltransferase II (CPT2). D. Aldolase B (ALDOB). E. Lysosomal beta-glycosidase (GBA). 39. Which one of the following combinations of laboratory findings is most likely to be observed in ethylene glycol intoxication? A. Decreased pH, increased serum osmolality and osmolal gap, decreased anion gap. B. Increased pH, decreased serum osmolality and osmolal gap, increased anion gap. C. Decreased pH, increased serum osmolality and osmolal gap, normal anion gap. D. Decreased pH, increased serum osmolality and osmolal gap, increased anion gap. E. Normal pH, increased serum osmolality and osmolal gap, increased anion gap. Major points of discussion ■ The high serum osmolality causes an efflux of cellular water, diluting the serum sodium. ■ Hyperkalemia results from red cell buffering of the acidosis. ■ Urine Na concentrations should be relatively normal in the setting of DKA, assuming renal function is uncompromised. ■ “Pseudo” is used to describe the hyponatremia seen in DKA because total body sodium stores are normal in the setting of low serum sodium. 2. A. Diagnose cystic fibrosis based on this chloride level and clinical symptoms. Major points of discussion ■ In addition to cystic fibrosis, several disorders, such as anorexia nervosa, atopic dermatitis, and protein-calorie malnutrition, can cause an elevated sweat chloride result. Sweat chloride measured after treatment of these conditions usually results in a normal chloride level. ■ A significant problem with sweat collection is that the amount of sweat collected may not be sufficient for chloride analysis. The Cystic Fibrosis Foundation recommends that if sweat is collected properly, 95% of the patients tested should have sufficient sweat for chloride analysis. ■ Technical problems associated with the collection of sweat and determination of chloride include skin contamination by salt-containing compounds, evaporation of the sweat during collection, errors in chloride determination, and errors in the calculation of sweat chloride results. ■ The collection of sweat and measurement of sweat chloride are manual procedures that are subject to technical errors. Only skilled technologists, who are trained in the collection of sweat and measurement of sweat chloride, should be used for these assays. ■ Sweat collection is usually not performed in newborns less than 48 hours after birth because the chloride values can be transiently elevated.7,40 3. A. Ion-selective electrode (ISE) with a potentiometric end point. Major points of discussion ■ The ISE method measures the potential that develops across a selective membrane. The low concentrations of chloride in sweat limit the use of the ISE method. ■ The mercuric ferric thiocyanate spectrophotometric method is usually not used for sweat chloride analysis because it is not sensitive at low chloride concentrations. ■ Isotopic dilution mass spectrometry is the ability to quantify a compound relative to an isotope species (in this case 37Cl) of known concentration. This is a manual procedure that is time consuming and not used in the routine clinical laboratory. ■ Measurement of sweat chloride levels is usually a manual procedure.7,40 4. A. Measurement of sweat osmolality. Major points of discussion ■ The Cystic Fibrosis Foundation requires quantitative sweat chloride measurements to be used to confirm the diagnosis of cystic fibrosis. A sweat chloride level of 60 mmol/L or greater is suggestive of cystic fibrosis. A chloride level between 40 and 59 mmol/L is an intermediate level, and a chloride level of 39 mmol/L or less is considered normal. ■ The Cystic Fibrosis Foundation suggests that two positive sweat chloride results be obtained to confirm the diagnosis of cystic fibrosis. An initial positive sweat chloride result should trigger the collection of another sweat sample and measurement of sweat chloride. ■ Almost all children with cystic fibrosis (99%) have elevated sweat chloride values. ■ Serum chloride levels are usually normal in cystic fibrosis patients.7,40 5. A. Increased pH, Po2, and Pco2. Major points of discussion ■ The longer the specimen is kept at room temperature, the more lactate is produced, and the lower the pH will be. ■ Glucose can be converted to CO2 when pyruvate enters the tricarboxylic acid cycle and is metabolized to CO2 and water. Leukocytes will metabolize oxygen and a falsely low Po2 will be obtained. ■ Because of glycolysis, a blood gas sample kept at 37 °C for 1 hour will result in a lower pH of approximately 0.04 to 0.08, a lower Po2 of approximately 5 to 10 mm Hg, and an increased Pco2 of approximately 5 mm Hg. Therefore, the sample should be placed on ice if analysis cannot be performed soon after obtaining the blood sample from the patient. ■ If the white blood cell count is high, glycolysis and its effect on pH, Pco2, and Po2 will be significantly exacerbated.31 6. A. Increased Po2, pH, and Pco2. Major points of discussion ■ Room air contains a Pco2 of essentially zero and a Po2 of approximately 150 mm Hg. Therefore, extraneous air in a blood sample will cause a diffusion of CO2 out of the specimen and O2 into the specimen (if the Po2 in the patient sample is actually < 150 mm Hg). ■ If the Po2 in the patient sample is actually greater than 150 mm Hg, which can occur in patients receiving oxygen therapy, the Po2 will diffuse out of the specimen and a lower Po2 will be obtained. The error will be greater as the amount of time the blood sample is exposed to room air increases. ■ The Pco2 of blood exposed to air will decrease and the pH, which is dependent on the HCO3–/0.03 Pco2 ratio, will increase. ■ If the Po2 is greater than 110 mm Hg and the patient is not being given oxygen, air contamination of the sample should be considered. The reference range for Po2 in an arterial blood sample is 80 to 100 mm Hg and, for a venous blood sample, it is 30 to 50 mm Hg.24 7. A. Online dialysis to remove proteins. Major points of discussion ■ The Jaffe method is used to measure creatinine. It was first described in 1886 and is the oldest clinical chemistry method routinely used in the clinical laboratory. ■ Noncreatinine substances can also react with picric acid but produce color at a slower or faster rate than creatinine. A significant number of substances in serum, other than creatinine, react with picric acid, causing positive interference. ■ The positive interferences from acetoacetate, pyruvate, acetone, protein, cephalosporins, glucose, uric acid, and ascorbic acid observed with the Jaffe reaction can be eliminated or significantly reduced using a kinetic rate procedure for measuring creatinine. Using this approach, these interfering analytes do not significantly react with picric acid during the 20- to 60-second time interval during which the measurements are taken.41 8. A. 32 mg/dL and 11.4 mmol/L. Major points of discussion ■ Traditionally, urea concentration is still calculated in terms of the urea nitrogen when expressed on a mass basis. ■ However, in SI (International System) units, the urea nitrogen is expressed as mmol/L of the intact molecule.19 9. A. Higher imprecision using the blood gas analyzer. Major points of discussion ■ Samples that are high in protein and/or lipids are considered “abnormal” and may result in an interference of measurement when dilution of samples is required. This occurs in indirect methods. ■ Indirect measurement methods typically require dilution of the sample before measurement. This is the most common method used with ion-specific electrodes on automated laboratory analyzers. ■ Direct measurement methods, such as those used on blood gas analyzers, do not require sample dilution before introduction to the ion-specific electrode. ■ A discrepancy can result between blood gas analyzers and automated analyzers if samples with high protein and/or high lipid levels are measured. This may cause confusion for ordering physicians who use this information for adjusting, initiating, and/or stopping fluid therapy. 10a. A. Decreased intact parathyroid hormone (PTH), increased calcium. 10b. A. Decreased intact PTH, increased calcium. 10c. A. Decreased intact PTH, increased calcium. 10d. A. Decreased intact PTH, increased calcium. Major points of discussion ■ The PTH molecule is a short polypeptide composed of 84 amino acids and has a short half-life in blood of 5 minutes. ■ Multiple assays are available for PTH. These include the intact PTH assay and separate assays for the biologically active amino-terminal portion of the molecule, internal PTH fragments, and the carboxyl-terminal portion of the molecule. It is important to know which assay is used for measurement of PTH. ■ The intact PTH assay detects the entire molecule and is the preferred assay for measuring this molecule because it provides excellent overall sensitivity and specificity. ■ The assays for PTH fragments provide improved sensitivity for the detection of disease but can be elevated under other conditions, including compromised kidney function. 11. A. Carboxyhemoglobin and deoxyhemoglobin. Major points of discussion ■ The total hemoglobin (Hb) level is composed of the oxy-, deoxy-, met-, and carboxy- forms of Hb. ■ Oxy- and deoxy- forms of Hb are functional because they are able to bind oxygen and carbon dioxide. ■ MetHb and carboxy Hb are nonfunctional with regard to oxygen-binding capacity. ■ So2 corresponds to the percentage of Hb that is bound to oxygen, relative to the amount of functional Hb (oxy + deoxy Hb). ■ Fo2 corresponds to the percentage of Hb that is bound to oxygen, relative to the amount of total Hb (functional + nonfunctional forms). 12. A. Elevated serum and urine levels of calcium and inorganic phosphate. Major points of discussion ■ Facts about primary hyperparathyroidism: • Defined as an increased secretion of PTH by abnormal parathyroid glands. • Incidence is 1:1000, with a female/male ratio of 3:1. • Peak incidence occurs at 30 to 60 years of age. • Symptoms are often vague and include the following: • In approximately 85% of the cases, it is caused by a single, benign, parathyroid adenoma. ■ PTH is an 84–amino-acid polypeptide with a molecular mass of 9425 Da that is synthesized and secreted by the chief cells of the parathyroid gland. Calcium homoeostasis is maintained by the actions of PTH on the bone, kidney, and indirectly in the intestine. • In the kidney, PTH increases renal tubular reabsorption of calcium, decreases tubular reabsorption of phosphate, and increases the excretion of phosphate. • In the intestine, PTH increases the absorption of dietary calcium by increasing the renal synthesis of 1,25-dihydroxyvitamin D, which stimulates intestinal absorption of calcium and phosphate. ■ Laboratory parameters in primary hyperparathyroidism • Elevated serum PTH levels resulting from increased secretion by the abnormal parathyroid glands. • Elevated serum calcium levels caused by the actions of PTH. • Low serum inorganic phosphate levels caused by increased renal phosphate excretion. • Increased urine levels of calcium and inorganic phosphate caused by increased secretion. ■ The treatment of primary hyperparathyroidism involves surgical removal of the hypersecreting parathyroid glands. Intraoperative PTH measurements are used to determine the success or failure of the procedure. The rationale for using PTH measurements to monitor parathyroid surgery is that PTH is produced only in the parathyroid glands. PTH has a short circulating half-life in patients with normal renal function; the intact PTH molecule has a half-life of less than 5 minutes. A rapid 50% decrease in PTH concentrations is observed if all hyperplastic/adenomatous parathyroid tissue has been removed. The secretion of PTH by the abnormal gland suppresses PTH secretion by the nearby normal parathyroid glands. After removal of the hyperplastic/adenomatous tissue, the normal glands begin to secrete PTH at appropriate levels.2,20 13. A. Plasma potassium. Major points of discussion ■ Osmolality is regulated by changes in water balance, with hyperosmolality stimulating secretion of ADH. ■ Sodium levels are also regulated by blood volume, primarily through the renin-angiotensin-aldosterone system. ■ The osmolality of plasma (in mmol/L) can be estimated by the equation 2 × [Na+]. ■ Approximately 75% of the NaCl in plasma is osmotically active. Therefore, 1 mole of NaCl dissociates into 0.75 mol Na+ + 0.75 mol Cl– + 0.25 mol NaCl. 14. A. Elevated circulating chloride and glucose levels. Major points of discussion ■ The dilution-based ISE method determines the concentration of sodium in the total sample volume, which includes dissolved solids as well as water. However, the clinically relevant sodium value is the plasma water concentration, not the total plasma concentration. ■ In the dilution method, sodium is calculated based on the assumption that 93% of plasma is water. However, in cases of severe hyperlipidemia, or in patients with large amounts of monoclonal proteins or with polyclonal gammopathies, the dissolved solids will increase and the percentage of plasma water will decrease. ■ When there is a decrease in plasma water, the calculated plasma sodium concentration will decrease. For example, if the serum protein concentration is 8.0 g/dL and the triglyceride concentration is 6.0 g/dL, the dissolved solids will comprise 14 g of every 100 g of plasma; this is equivalent to 14%, resulting in a water content of 86%. In this case, the sodium concentration will be decreased by 86/93, or 8%. This condition is termed “pseudohyponatremia” and is observed in patients with hyperlipidemia or with elevated protein concentrations. ■ Specimens with elevated concentrations of lipids or proteins do not affect the direct ISE methods because they measure the sodium concentration directly in plasma and make no assumption about the water content. 15. A. Congestive heart failure. Major points of discussion ■ Although blood urea concentration increases as glomerular filtration declines, urea is a poor marker for kidney disease because urea production rates are not constant and depend on the activity of the urea cycle enzymes and the protein load. ■ Urea, as BUN, is usually measured along with creatinine. Therefore, the BUN/creatinine ratio may be used as a diagnostic tool. The normal serum or plasma BUN/creatinine ratio is 10 to 20. A low BUN/creatinine ratio is seen in patients following a low-protein diet and in severe liver disease because of the reduced synthesis of urea. ■ In a healthy individual, a high-protein diet will result in an increased synthesis of urea, resulting in a high BUN/creatinine ratio. ■ In renal tubular injury, the BUN and creatinine increase in parallel and a normal BUN/creatinine ratio is often maintained.19 16. A. The calculation requires the serum creatinine and urine creatinine levels along with patient age, gender, and race. Major points of discussion ■ GFR is used to estimate kidney function. A decrease in GFR precedes kidney failure. A GFR value of more than 60 mL/min/1.73 m2 is considered normal. ■ The MDRD equation was validated against an iothalamate reference method for estimating the GFR. The GFR calculated by the MDRD equation is more accurate than the GFR calculated from the creatinine clearance, and it is the most commonly used equation in the United States for estimating the GFR. ■ A limitation of the MDRD equation is that it shows a negative bias at high GFR values. Therefore, it has been recommended that the numerical value of the GFR calculated using the MDRD equation be reported only up to 60 mL/min/1.73 m2. ■ An equation from the Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) study, which is based on the log serum creatinine concentration along with age, gender, and race, has also been used to calculate the GFR. The CKD-EPI equation more accurately categorized the risk of kidney disease and mortality than the MDRD equation. Many other equations have been derived that estimate the GFR. ■ Serum creatinine level is not a perfect marker for GFR. Tubular secretion, diet, drugs, muscle mass, and analytical interferences affect the creatinine concentration.22 17. A. Metabolic acidosis with an elevated anion gap. Major points of discussion ■ In addition, ingestion of toxic compounds that are metabolized to acid metabolites (e.g., methanol is converted to formic acid; ethylene glycol is converted to oxalic acid) will also result in metabolic acidosis. ■ Metabolic organic acids react with plasma HCO3− to form carbonic acid, which is converted to CO2 gas and is eliminated from the body by ventilation. In this setting, there is a decrease in HCO3− concentration with little or no loss of Pco2. ■ The anion gap is the difference between the measured cations and the measured anions. The anion gap is calculated as [measured cations (Na + K)] – [measured anions (Cl + HCO3)]. The reference range is 7 to 16 mmol/L. ■ In the case described in this question, the anion gap is 25 mmol/L. An elevated anion gap is observed when organic acids cause the acidosis. ■ In metabolic acidosis, HCO3− is decreased and Pco2 can be normal or slightly decreased. The HCO3−/0.03 Pco2 ratio, which is used in the Henderson-Hasselbalch equation to calculate the pH, is decreased, thereby causing the low pH. ■ Compensation of metabolic acidosis is achieved by hyperventilation, which decreases the Pco2 and increases the HCO3−/0.03 Pco2 ratio, thereby helping to increase the pH.25 18. A. Metabolic alkalosis with an elevated anion gap. Major points of discussion ■ In severe diarrhea, in addition to HCO3−, there is a loss of Na+ and K+ as part of the watery stool. As HCO3– is lost, chloride ions are reabsorbed along with Na+ or K+ to maintain electrical neutrality. If the water, K+, and HCO3− in the intestine are not reabsorbed, hypokalemia, along with a normal anion gap, will occur. ■ The HCO3−/0.03 Pco2 ratio, which is used in the Henderson-Hasselbalch equation to calculate the pH, is decreased resulting in a low pH. ■ In diarrhea, although chloride ions may be elevated, the anion gap is in the normal range. ■ In severe diarrhea, if there are no additional organic acids present (e.g., lactic acid and acetoacetic acid), the anion gap will be normal.25 19. A. Metabolic acidosis. Major points of discussion ■ Hypovolemia (e.g., from severe vomiting) will result in reabsorption of Na+ to restore volume, and the renal retention of HCO3− in the presence of low chloride concentrations to maintain electrical neutrality. The decrease of chloride in metabolic alkalosis is known as hypochloremic alkalosis. ■ In addition, K+ and H+ are excreted in exchange for Na+, which will increase the blood pH. The urine pH may be acidic in metabolic alkalosis. ■ The anion gap is the difference between the measured cations and the measured anions. The anion gap is calculated as [measured cations (Na + K)] – [measured anions (Cl + HCO3−)]. The reference range is usually 7 to 16 mmol/L. In the case in this question, the anion gap was 9 mmol/L. A normal anion gap is usually observed in metabolic alkalosis. ■ In metabolic alkalosis, HCO3− is increased, Pco2 is normal or slightly increased, and the HCO3−/0.03 Pco2 ratio, which is used in the Henderson-Hasselbalch equation to calculate the pH, is increased, which causes the elevated blood pH.14,25 20. A. Metabolic alkalosis with respiratory compensation. Major points of discussion ■ The increase in blood pH depresses the respiratory center. The physiological response to metabolic alkalosis is hypoventilation, which raises the Pco2 (partial pressure of carbon dioxide) and HCO3− concentrations in the blood. ■ The rise in Pco2 is greater than the increase in HCO3−. As a result, the HCO3−/0.03 Pco2 ratio is decreased, which lowers the pH. However, the actual concentrations of both HCO3−and Pco2 remain elevated. ■ The result of this physiological compensation is a pH level that is at, or close to, the reference range in the presence of an elevated HCO3− level. ■ This physiological mechanism for adjusting the pH during metabolic alkalosis is termed compensatory respiratory acidosis.25 21. A. Predicts glomerulonephritis. Major points of discussion ■ Diabetic nephropathy is the leading cause of chronic renal failure in the United States. The number of patients with diabetic nephropathy reflects the increase in the prevalence of obesity, metabolic syndrome, and type 2 diabetes in the United States. ■ Microalbuminuria is an indicator of deteriorating renal function in diabetic patients and is the first abnormal biochemical marker to appear in diabetic nephropathy. Microalbuminuria occurs because of structural changes in the glomerular filtration barrier, which cause leakage of albumin. ■ Elevated levels of albumin in urine appear before there is a decrease in the glomerular filtration rate. In diabetic patients, microalbuminuria is also an important risk factor for predicting the development of cardiovascular disease. ■ Patients with diabetes should have their microalbumin levels measured at least once per year. If an elevated microalbumin level is obtained, other risk factors (hypertension, obesity, and so forth) should be controlled to prevent or delay the onset of renal damage.3,35 22. A. Increased serum BUN and creatinine concentrations. Major points of discussion ■ Nephrotic syndrome can be caused by primary kidney disease, such as focal segmental glomerulosclerosis and membranous nephropathy, or can result from secondary causes, such as diabetes mellitus and infections. Renal biopsy plays a major role in the evaluation of patients with nephrotic syndrome. ■ The “nephrotic pattern” results from the loss of albumin and other low-molecular-weight serum proteins through the damaged nephron. ■ Physiological attempts to compensate for nephrotic syndrome by restoring oncotic pressure include increasing the synthesis of large-molecular-weight proteins, such as α2-macroglobulin and lipoproteins. Dyslipidemia results in an increase in total cholesterol and low-density lipoprotein cholesterol, which contributes to an increase in cardiovascular mortality in these patients. ■ The reduction of circulating levels of albumin caused by renal loss, accompanied by decreased urine output, ultimately leads to edema.18,32 23a. A. Renal artery ultrasound. 23b. A. 6 g/dL, 0.99 g/dL, 4 g/dL. Major points of discussion ■ In renal tubular disease, phosphate excretion is prevented because of nonresponsiveness to PTH. ■ This hyperphosphatemia, along with defective vitamin D regulation, contributes to hypocalcemia. ■ The ionized calcium is a reflection of the biologically available calcium and should also be low. ■ A normal ratio of urine to serum osmolality implies properly functioning tubules and suggests glomerular injury. ■ A low urine/serum osmolality (< 1.2 in a fluid restricted sample) suggests the primary concentration mechanism localized in the tubules is defective. 24. A. Metabolic acidosis with a normal anion gap. Major points of discussion ■ An elevated Pco2 level characterizes all causes of respiratory acidosis. The physiological response to respiratory acidosis includes renal excretion of acids and chloride and retention of sodium and HCO3−. ■ The anion gap is the difference between the measured cations and the measured anions. The anion gap is calculated as [measured cations (Na + K)] – [measured anions (Cl + HCO3−)]. The reference range is 7 to 16 mmol/L. ■ In the case described in this question, the anion gap was 12 mmol/L. A normal anion gap is usually observed in respiratory acidosis. ■ In respiratory acidosis, there is an increase in both Pco2 and HCO3−. However, the increase in HCO3− is less than the increase in Pco2, and the HCO3−/0.03 Pco2 ratio, which is used in the Henderson-Hasselbalch equation to calculate the pH, is decreased, thereby leading to a low pH.25 25. A. Metabolic acidosis. Major points of discussion ■ Respiratory alkalosis can be caused by pulmonary disorders such as pneumonia, pulmonary embolism, interstitial lung disease, and pulmonary fibrosis. Nonpulmonary causes of respiratory alkalosis include anxiety, hysteria, fever, hypoxia, and drugs. ■ The anion gap is the difference between the measured cations and the measured anions. The anion gap is calculated as [measured cations (Na + K)] – [measured anions (Cl + HCO3−)]. The reference range is 7 to 16 mmol/L. In the case described in this question, the anion gap was 12 mmol/L. A normal anion gap is usually observed in respiratory alkalosis. ■ In respiratory alkalosis, the Pco2 is decreased and the HCO3−/0.03 Pco2 ratio, which is used in the Henderson-Hasselbalch equation to calculate the pH, is increased. Respiratory alkalosis is associated with a decreased Pco2, an increased pH, and a low HCO3− because of renal compensation. ■ Individuals living at high altitudes chronically hyperventilate because of hypoxia and typically have a lower Pco2 than individuals living at sea level.25 26. A. Renal regulation of Pco2. Major points of discussion ■ In respiratory alkalosis, two types of physiological compensation are typically used to lower the HCO3− concentration;:tissue buffering and renal compensation. ■ Compensation by tissue buffering of HCO3− involves both erythrocytes and tissue buffers, which provide H+ to react with HCO3− to produce carbonic acid, which is then converted to CO2 and water. The CO2 is removed by hyperventilation. ■ Renal compensation in respiratory alkalosis occurs when the kidneys excrete increased amounts of HCO3−. The proximal tubules of the kidney also contribute to this effect by decreasing the reabsorption of HCO3−. The renal response to respiratory alkalosis is termed “compensatory metabolic acidosis.” ■ Metabolic compensation is very effective in respiratory alkalosis and, in some cases, the pH returns to a normal level. In respiratory alkalosis,25 alkalinization of the urine may occur as a result of the increased circulating HCO3−. 27. A. Low levels of calcium, 25-hydroxyvitamin D, inorganic phosphate, and PTH. Major points of discussion ■ Vitamin D is synthesized in the skin when ultraviolet B (UVB) radiation from the sun reacts with 7-dehydrocholesterol to form vitamin D3, which is metabolized in the liver to 25-hydroxyvitamin D3 and in the kidney to the active metabolite 1,25-dihydroxyvitamin D3. Vitamin D can also be obtained from foods, such as fatty fish, or from vitamin supplements. ■ Vitamin D is a hormone that increases the intestinal absorption of calcium and inorganic phosphate into the blood. Without sufficient levels of vitamin D, only approximately 10% of dietary calcium and 60% of dietary phosphate are released into the circulation by passive diffusion. The stimulation of active transport of calcium and phosphate from the intestine into the blood by vitamin D accounts for approximately 40% of dietary calcium and 80% of dietary phosphate absorption. ■ In rickets, low levels of serum calcium, phosphate, and 25-hydroxyvitamin D, and elevated levels of PTH are seen. The low serum calcium level triggers the release of PTH from the parathyroid glands. PTH acts on osteoclasts to release calcium and phosphate from bone to help maintain normal calcium levels. Therefore, in rickets, the bones are soft and weak, which results in the skeletal deformities seen in these children. ■ In the United States, fortification of foods with vitamin D has significantly reduced the incidence of rickets. However, rickets is commonly seen in developing countries when the mother is vitamin D deficient and breastfeeding is the major source of infant nutrition.12,13 28. A. Oxyhemoglobin and carboxyhemoglobin. Major points of discussion ■ Although So2 is a measurement of functional hemoglobin that is bound to O2, Po2 is more commonly used in assessing oxygen-binding capacity in the lungs. ■ Decreased Po2 can be caused by decreased pulmonary ventilation, impaired gas exchange, or altered blood flow between the heart and lungs. ■ Carboxyhemoglobin contains carbon monoxide (CO) bound to hemoglobin; carbon monoxide has a much higher affinity for hemoglobin than O2. ■ Methemoglobin contains iron in its ferric (Fe3 +), not ferrous (Fe2 +), state and, therefore, cannot bind oxygen or carbon dioxide. 29. A. Anticoagulant contamination. Major points of discussion ■ Potassium-EDTA contamination is common and may still result in normal levels of potassium, calcium, and magnesium. This could potentially mask dangerous hypokalemia. In this case, measurement of EDTA itself is critical to identify the source of spurious results. ■ Mechanisms of contamination include backflow from vacuum tubes if EDTA tubes are used before others, decanting from EDTA tubes into others, and needle contamination when EDTA tubes are used first. ■ Other examples of spurious hyperkalemia include contamination from intravenous potassium infusion, prolonged tourniquet time with fist clinching, in vitro hemolysis, release from leukocytes or platelets, and cold storage of the sample before serum separation. ■ Causes of true hyperkalemia include decreased renal elimination (kidney insufficiency, hypoadrenalism, type 4 RTA, potassium-sparing diuretics, cardiac failure, cirrhosis), cell lysis (rhabdomyolysis, tumor lysis syndrome, massive blood transfusion), or excessive potassium administration (diet or intravenous, usually in combination with one of the above).4,34,37 30a. A. Addison disease. 30b. A. Ectopic adrenocorticotropic hormone (ACTH) production. 30c. A. Unchanged cortisol after ACTH administration. Major points of discussion ■ ADH or arginine-vasopressin (AVP) is synthesized in the supraoptic and paraventricular nuclei of the hypothalamus, packaged into neurosecretory granules, which then migrate to the posterior pituitary. Secretion occurs predominantly in response to higher plasma osmolality sensed by osmoreceptors in the hypothalamus. Central diabetes insipidus results from damage to the hypothalamic-pituitary axis (e.g., surgery, tumors, trauma, vascular malformations, infarction, infectious, granulomatous, and inflammatory diseases). In this patient, neurosarcoidosis should be investigated as a possible cause of central diabetes insipidus. In children, congenital defects in ADH synthesis, storage, and release can cause central diabetes insipidus. ■ Nephrogenic diabetes insipidus results from a tubular defect in the response to ADH, which is mediated by the ADH receptor (AVPR2), a G protein–coupled receptor present in the basolateral membrane of the collecting duct. Binding of ADH to AVPR2 results in activation of GαS, adenylcyclase, protein kinase A, and transport of the water channel aquaporin 2 (AQP2) present in intracellular vesicles to the apical surface of the collecting ducts. Water reabsorption from the tubular lumen is initiated by flow to the intracellular space through AQP2, followed by exit to the basolateral side through AQP3 and AQP4. ■ Mutations in either AVPR2 or AQP2 can cause congenital nephrogenic diabetes insipidus. Acquired defects include drugs (lithium, cisplatin, foscarnet), postacute tubular necrosis, postobstruction diuresis, osmotic diuresis, and electrolyte disturbances, including hypokalemia and hypercalcemia. High levels of calcium reduce the response to ADH in the tubule, perhaps by interfering with AQP2 expression and movement to the apical surface, in addition to causing polydipsia. ■ Two other forms of diabetes insipidus include primary polydipsia, where psychogenic increases in water intake result in lower plasma osmolality and suppressed ADH production, and gestational diabetes insipidus, resulting from excessive metabolism of ADH by placental enzymes. ■ The various forms of diabetes insipidus can be distinguished by performing a water deprivation test, which should be performed with close supervision given the risk of dehydration and hypovolemia. Plasma and urine osmolality is measured hourly, and the test is stopped when the urine osmolality stops increasing or the plasma osmolality exceeds 300 mOsm/kg. ADH is administered to assess the ability of the kidneys to respond. In both central and nephrogenic diabetes insipidus, the urine does not concentrate more than plasma in response to water deprivation, in contrast to psychogenic polydipsia. After ADH administration, the urine osmolality does not increase by more than 10% in nephrogenic diabetes insipidus, but it will increase by more than 50% in central diabetes insipidus. ■ Sarcoidosis can cause diabetes insipidus by infiltration of the hypothalamic pituitary axis or by production of 1,25-dihydroxyvitamin D (calcitriol) from granulomatous macrophages expressing CYP27B1. Excessive production of calcitriol will result in increased absorption and bone mobilization of calcium and phosphorus, with resulting suppression of PTH and PTHrP.23,30,42 31. A. Rhabdomyolysis secondary to blood hyperviscosity. Major points of discussion ■ Pseudohypokalemia is seen with ambient temperatures higher than 30°C in samples when separation of plasma has been delayed. Seasonal variations in the proportion of hypokalemia have been reported, with frequency peaking in hot summer periods. With continued metabolic activity at temperatures higher than 30°C, consumption of glucose can lead to cell death and release of potassium from leukocytes, potentially resulting in spurious hyperkalemia. ■ In contrast, pseudohyperkalemia can be seen when whole blood is incubated at lower temperatures, which inhibit the cellular metabolism required for sodium/potassium exchange, resulting in a rise of about 0.2 to 0.3 mmol/L/hr at 4°C. It is also seen in cases of leukocytosis with fragile cells (e.g., chronic lymphocytic leukemia), particularly during clotting, resulting in potassium levels higher in serum than in plasma. In some cases, reverse pseudohyperkalemia is seen when heparin induces release of potassium from leukemic cells into the plasma. ■ Given the potential for misleading potassium levels (hypokalemia and hyperkalemia) in patients with high leukocyte counts, it is recommended that whole blood samples be rapidly centrifuged with a gel separator. If centrifugation must be delayed, incubation on ice may prevent spurious hypokalemia but should not exceed 1 hour to avoid pseudohyperkalemia. ■ In some patients with acute leukemia, true hypokalemia occurs as a result of leukemic nephritis with renal potassium wasting. Therefore, careful attention to pre-analytical factors is essential for the correct management of these patients.21,27,36 32. A. The normal BUN/creatinine ratio is inconsistent with dehydration. Major points of discussion ■ Marked hyperglycemia induces fluid shifts to the extravascular compartment and osmotic diuresis, resulting in intracellular hyperosmolality and dehydration. ■ The BUN/creatinine ratio reflects production of urea and creatinine, as well as the GFR (reflected by creatinine levels) and the degree of urea reabsorption in the proximal tubule. With severe hyperglycemia, osmotic diuresis occurs with high urine flow and decreased urea reabsorption. With adequate water intake, the BUN/creatinine ratio can be normal or even low in situations of high osmotic diuresis, despite significant intracellular dehydration. While osmotic diuresis depletes the extracellular compartment of water, the BUN/creatinine ratio increases in proportion to the degree of dehydration. Most patients with HHS present with dehydration and an elevated BUN/creatinine ratio. ■ The measurement of sodium truly reflects its concentration in the extravascular fluid but it should be appropriately corrected by the degree of hyperglycemia to estimate total body sodium. This should not be confused with pseudohyponatremia caused by reduced plasma water (e.g., with hypertriglyceridemia, hyperproteinemia) when indirect ion-specific electrodes make these measurements. ■ The actual plasma sodium concentration reflects the balance of water distribution between the extracellular and intracellular compartments, as well as between water and sodium intake and losses. In HHS, the average sodium is 144 mmol/L, but it can be low (as in this patient) or elevated, especially because water losses tend to be higher than sodium losses as a result of osmotic diuresis. In virtually all cases of HHS, the total body sodium (and potassium) stores are depleted because of large renal losses, even when plasma concentrations are normal or high.17 33. A. Type 1 diabetes complicated by urinary tract infection. Major points of discussion ■ The clinical presentation of ketoacidosis with high anion gap points to organic acidemia. The most important causes of organic acidemia resulting from an IEM are methylmalonic acidemia and the related propionyl acidemia. Propionyl-CoA is derived from the metabolism of isoleucine, valine, methionine, threonine, and thymine, as well as cholesterol and odd-chain fatty acids and is converted to methylmalonyl-CoA by propionyl-carboxylase. ■ Vitamin B12 is metabolized to methylcobalamin and adenosylcobalamin. Adenosylcobalamin is required for the mitochondrial enzyme methylmalonyl CoA mutase (MUT), which isomerizes methylmalonyl-CoA to succinyl-COA. Defects in MUT or adenosylcobalamin formation result in methylmalonic acid accumulation. Methylcobalamin is required for the cytoplasmic enzyme methionine synthase, which adds a methyl group to homocysteine to form methionine. In isolated deficiencies of methylcobalamin formation, levels of homocysteine would be increased. In deficiencies of cobalamin, such as pernicious anemia or nutritional vitamin B12 deficiency, both methylmalonic acid and homocysteine would be elevated. ■ Patients with methylmalonic or propionic acidemia typically present with hypoglycemia, possibly caused by inhibition of gluconeogenesis by propionate, methylmalonate, or their acyl-CoA derivatives. However, acute decompensation with severe ketoacidosis and hyperglycemia can be a rare presentation. In some of these cases, the hyperglycemia responds to insulin therapy and treatment of the underlying precipitating factor, such as infection. In other cases, lack of response to insulin treatment points to an IEM as a possible cause. ■ Measurement of C3-acylcarnitine (propionyl-carnitine) by tandem mass-spectrometry is used as a prenatal screening tool for propionyl or methylmalonyl acidemia. In these defects, backup flux leads to accumulation of propionyl-CoA and its conversion to propionyl-carnitine and expulsion from the mitochondria. ■ Secondary lactic acidosis results from depletion of succinyl-CoA, which leads to impaired oxidative phosphorylation and therefore increased anaerobic glycolysis with lactate accumulation. The lactate/pyruvate ratio increases in situations distal to pyruvate dehydrogenase, as is the case with impaired oxidative phosphorylation, while a normal ratio with increases in both pyruvate and lactate indicates a deficiency in pyruvate dehydrogenase or other steps of gluconeogenesis.6,10,33 34. A. A standard meal will decrease plasma phosphate levels within 2 hours. Major points of discussion ■ Postural effects can affect plasma electrolyte levels. Compared with recumbency, the upright position causes increased intravascular pressure and a flow of fluid from the intravascular to the extravascular compartment, resulting in about an 8% to 15% decrease in plasma volume and corresponding increase in hematocrit. With normal permeability, substances larger than 4 nm in diameter (such as proteins) are retained in the intravascular space, while water and electrolytes flow into the extravascular space. While most electrolytes are not complexed and equilibrate rapidly between the two compartments, the portion of total calcium that is protein bound (around 40%) is retained in the intravascular compartment and its concentration can increase by about 5% to 10%. Only 25% to 30% of magnesium is complexed with albumin, and therefore, increases only by about 3% to 5%. In addition, posture induces changes in the renin-angiotensin-aldosterone axis with corresponding effects in renal excretion of sodium and potassium. ■ Exercise can lead to increased release of intracellular ions such as potassium, phosphate, and magnesium. Although total calcium does not change with exercise, lactate production during intense exercise may cause pH-induced dissociation of protein-bound calcium and transient increases in ionized calcium. ■ Meals can cause transient increases in intracellular ions, particularly phosphate (up to 15%) and potassium (up to 5%), which are followed by insulin-stimulated intracellular influx and normalization within 2 to 3 hours. ■ Many drugs affect plasma electrolyte concentrations, particularly those affecting renal and cardiovascular function such as diuretics and angiotensin-converting enzyme inhibitors. ■ Ethanol intoxication can cause ketosis and metabolic acidosis. Excess protons in metabolic acidosis compete with potassium for intracellular influx and may result in hyperkalemia. ■ In addition to mineralocorticoids, various hormones affect electrolyte levels. For example, insulin and catecholamines induce intracellular influx of potassium and phosphate.15 35. A. Ethanol contamination during phlebotomy. Major points of discussion ■ Hemolysis is the most frequent cause of spurious hyperkalemia. Since the intracellular concentration of potassium is about 105 mmol/L in red cells, even a minor amount of hemolysis results in significant increases in plasma potassium. On average, potassium increases about 0.2 to 0.5 mmol/L per 0.1 g/dL of plasma hemoglobin. Hemolysis is typically visible with the naked eye when plasma hemoglobin is above 20 to 70 mg/dL. Potentially clinically significant spurious increases in potassium generally occur with hemoglobin levels above 100 mg/dL (corresponding to lysis of about 0.7% of the red cells with a hematocrit of 45%). ■ Causes of hemolysis include ethanol contamination, difficult phlebotomy, inappropriate needle diameter, collection with syringe and needle, inappropriate storage or transport temperature, transport via pneumatic tube, excessive centrifugation speed, and so on. A particular problem is spurious hyperkalemia during whole blood analysis, which does not benefit from visual or spectrometric detection of plasma hemoglobin. ■ Additional causes of spurious hyperkalemia include contamination with intravenous fluids high in potassium and with anticoagulants containing potassium, most frequently K2-EDTA, which will also cause spurious hypocalcemia. ■ In vitro release of potassium from other blood cells in the absence of hemolysis can also occur with thrombocytosis and leukocytosis, particularly with fragile leukocytes, such as in chronic lymphocytic and chronic myelomonocytic leukemia. The degree of spurious potassium elevation tends to be higher in serum than plasma because of cell lysis during clotting. However, in certain cases, reverse pseudohyperkalemia is seen, with heparin-induced lysis of leukemic cells and consequent higher levels in plasma than serum. ■ Prolonged application of a tourniquet, especially when coupled with fist clenching, can result in release of potassium from muscle without signs of hemolysis. Prolonged incubation of unseparated blood at refrigerated temperatures inhibits the sodium-potassium ATPase that maintains high levels of intracellular potassium. Similarly, prolonged incubation at temperatures higher than 30°C can lead to consumption of glucose and dysfunction of the ATPase. ■ Familial pseudohyperkalemia (FP) is caused by an autosomal dominant gene defect, leading to leakage of potassium at temperature less than 37°C. This defect is due to structural alterations in the red cell membrane that increases permeability at lower temperatures. Recent evidence implicates mutations in the ABCB6 transporter in FP. Similar defects with ion leakage are also seen in some hereditary stomatocytoses. Prompt separation of plasma from cells after blood collection is essential for accurate potassium measurements. This condition has no clinical significance other than erroneous interpretation of potassium levels, leading to inappropriate therapeutic intervention.1,37 36a. A. Organic aciduria. 36b. A. High urinary levels of methylmalonic acid by gas chromatography–mass spectrometry. Major points of discussion ■ Type 1 RTA is caused by insufficient H+ excretion at the distal tubule. Therefore, the kidney is unable to eliminate excessive acid and acidify the urine in situations of systemic acidosis. Causes include calcium-induced tubular damage, drugs (e.g., lithium, amphotericin B, nonsteroidal anti-inflammatory drugs), toxins (toluene), paraproteins, autoimmune disorders, and genetic defects in distal transporters (SLC4A1, ATP6V1B1, ATP6V0A4). Since acid excretion is reduced, sodium preferably exchanges with potassium at the distal tube, and hypokalemia can be present. In hyperkalemic type I RTA, the sodium load to the distal tube is reduced and therefore potassium secretion is impaired. This can result from any cause of marked volume depletion with enhanced proximal tubule reabsorption of sodium or from inhibition of distal sodium reabsorption with potassium-sparing diuretics, lithium, trimethoprim, sickle cell nephropathy, lupus nephritis, or urinary obstruction. ■ Type 2 RTA is caused by failure of the proximal tubule to reabsorb bicarbonate. When the distal tubule is unable to reabsorb all the excessive bicarbonate lost by the proximal tubule, bicarbonaturia results with a consequent decrease in plasma bicarbonate (metabolic acidosis) and contraction of the plasma volume, leading to increases in aldosterone secretion and hypokalemia. Although the plasma bicarbonate stabilizes at a lower level and the distal tubule increases bicarbonate reabsorption and acid secretion, this is a self-limiting condition and acidosis can be resolved. Type 2 RTA can result from an isolated defect of bicarbonate reabsorption, such as mutations in the NBC1 sodium/bicarbonate transporter or in carbonic anhydrase 2, or from a generalized impairment in the reabsorption of small molecules, including phosphate, glucose, uric acid, calcium, citrate, and amino acids. In generalized defects (Fanconi syndrome), the levels of these substances are reduced in the plasma. Causes include genetic defects, as well as proximal tubular damage or inhibition by nephrotic syndrome, amyloidosis, toxins, drugs, paraproteins, and biochemical defects (cystinosis, Wilson disease, methylmalonic acidemia, galactosemia, glycogen storage diseases, and so on). ■ Type 4 RTA is due to defects in mineralocorticoid production or response and manifests by hyperkalemia with hyperchloremic metabolic acidosis, usually in the setting of renal failure (diabetic or hypertensive nephrosclerosis, tubulointerstitial disease, acquired immunodeficiency syndrome). ■ NH3 is secreted by the distal tubule to bind H+ as NH4+, acidifying the urine. Ammonium can be indirectly estimated by calculating the urinary anion gap (UAG) = urNa+ + urK+ – urCl–. With extrarenal causes of metabolic acidosis, the kidney is able to increase NH4+ production, and the UAG becomes negative (due to the compensatory increase in Cl– excretion). In RTA, NH4+ production is reduced and the UAG is usually positive. ■ The fractionary excretion of bicarbonate is calculated by the following formula:
Clinical Chemistry
Electrolytes, Blood Gases, Renal Function
Which one of the following choices most likely represents her additional lab findings?
Rationale: This is an intermediate chloride level and is not diagnostic for cystic fibrosis.
B. Measure immunoreactive trypsinogen levels in the blood to confirm the diagnosis of cystic fibrosis.
Rationale: Immunoreactive trypsinogen is a screening test and cannot be used to diagnose cystic fibrosis.
C. Diagnose this individual as a cystic fibrosis carrier with a mutated cystic fibrosis transmembrane conductance regulator (CFTR) gene.
Rationale: An intermediate chloride level cannot be used to predict who is a carrier of cystic fibrosis.
D. Collect another sweat sample and repeat the chloride measurement.
Rationale: Intermediate sweat chloride levels require the collection of another sweat sample and measurement of chloride.
E. Measure serum sodium and chloride levels to confirm the diagnosis of cystic fibrosis.
Rationale: Serum sodium and chloride levels are not used to diagnose cystic fibrosis.
Rationale: This is the most frequent method used to measure chloride levels in serum or plasma.
B. Mercuric ferric thiocyanate-colorimetric method.
Rationale: This method is seldom used to measure sweat chloride.
C. Enzymatic measurement.
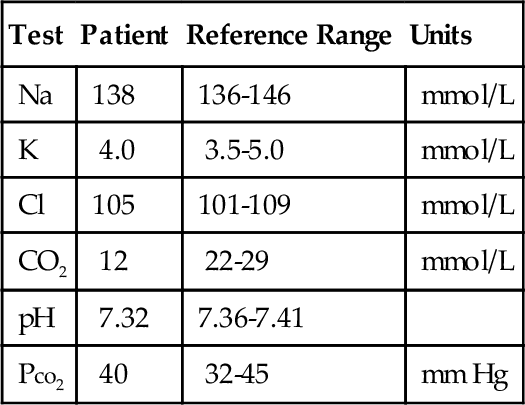
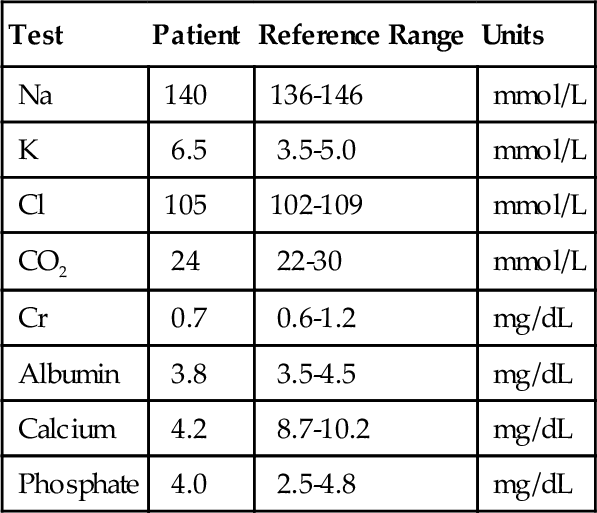
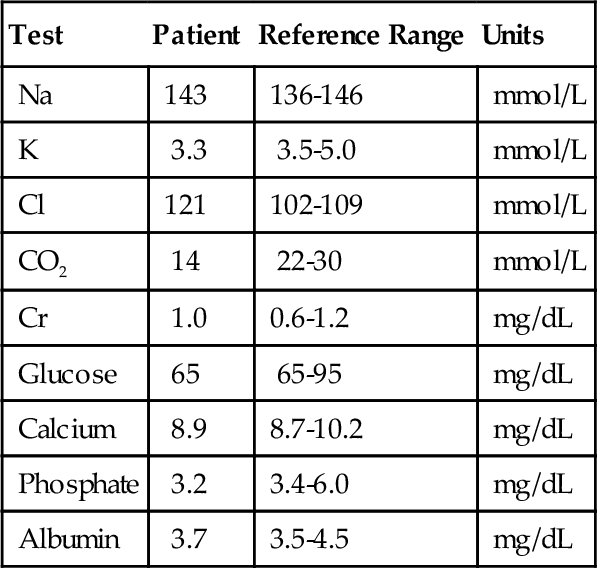
Rationale: This method is not available.
D. Coulometric titration procedure.
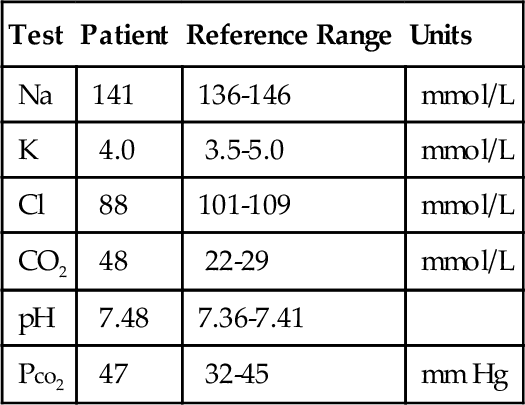
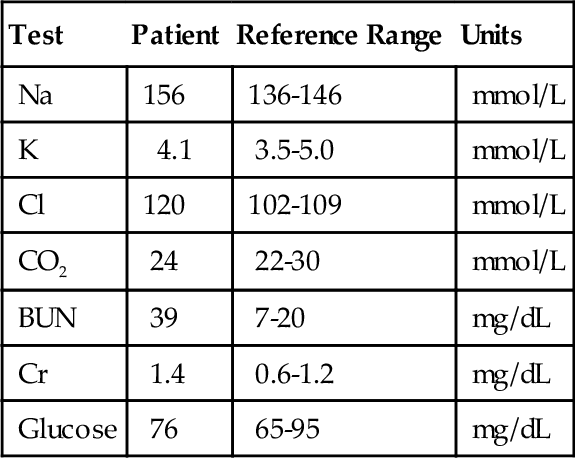
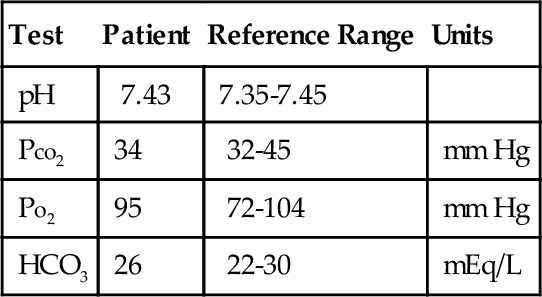
Rationale: This is the most common method used to measure sweat chloride and is recommended by the Cystic Fibrosis Foundation.
E. Isotopic dilution mass spectrometry method.
Rationale: This method is used in research laboratories.
Rationale: Sweat osmolality is a screening test for cystic fibrosis.
B. Measurement of immunoreactive trypsinogen.
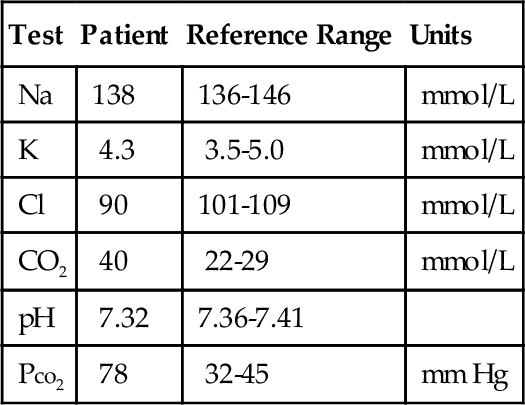
Rationale: Immunoreactive trypsinogen is a screening test for cystic fibrosis.
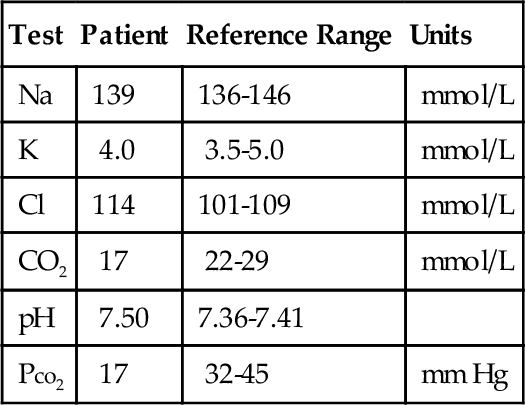
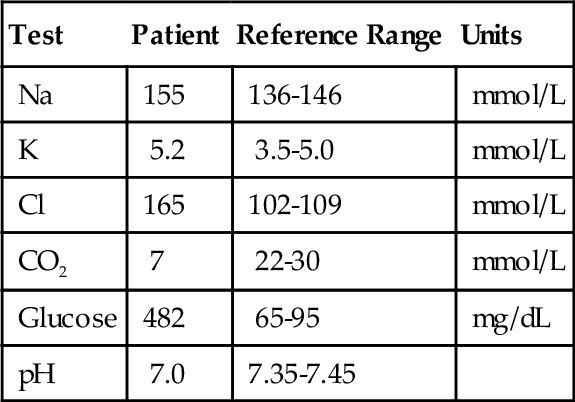
C. Measurement of sweat chloride.
Rationale: Quantitative measurement of sweat chloride is used to diagnose cystic fibrosis.
D. Measurement of sweat conductivity.
Rationale: Sweat conductivity measurement is a screening test for cystic fibrosis.
E. Measurement of serum chloride.
Rationale: Serum chloride levels are not used to diagnose cystic fibrosis.
B. Decreased pH and increased Po2 and Pco2.
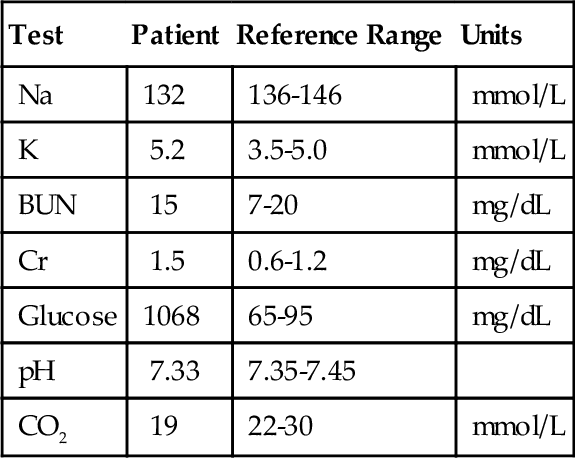
C. Decreased pH and Pco2 and increased Po2.
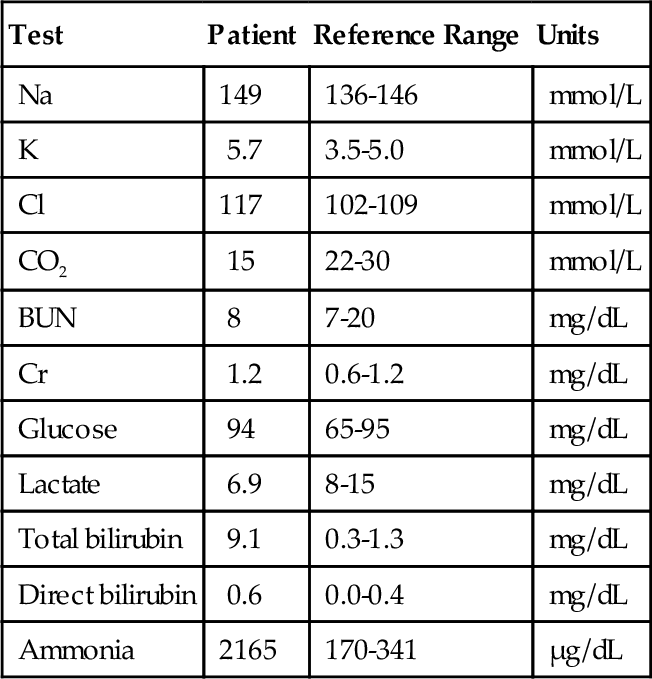
D. Decreased pH and Po2 and increased Pco2.
E. Decreased pH, Po2, and Pco2.
Rationale: Decreased pH and po2 and increased Pco2 will occur when glucose is metabolized to pyruvate and lactate.
B. Decreased Po2, pH, and Pco2.
C. Increased Po2 and pH and decreased Pco2.
D. Unchanged Po2 and decreased Pco2 and pH.
E. Decreased Po2 and pH and increased Pco2.
Rationale: An arterial blood sample exposed to air will lead to an increase in Po2 and pH and a decrease in Pco2.
Rationale: Dialysis is not performed.
B. Fluorescence measurement of the color produced.
Rationale: Fluorescence is not used in the automated Jaffe reaction.
C. Spectrophotometric measurement of the color produced at two different wavelengths.
Rationale: Bichromatic measurements are not used in the automated Jaffe reaction.
D. Spectrophotometric measurement of the color produced after the reaction has gone to completion.
Rationale: The color is not measured at the end point of the reaction.
E. Kinetic assay, where the reaction rate is measured between 20 and 60 seconds.
Rationale: The method involves the reaction of picric acid with creatinine in an alkaline solution to form an orange-red complex that is measured spectrophotometrically at a wavelength of 520 nm.
B. 32 mg/dL and 5.3 mmol/L.
C. 7 mg/dL and 5.3 mmol/L.
D. 7 mg/dL and 11.4 mmol/L.
E. 64 mg/dL and 15 mmol/L.
Rationale: The molecular weight of urea is 60. Urea contains 2 nitrogen atoms, with a combined molecular weight of 28. To convert from urea nitrogen in mg/dL to urea in mg/dL, divide 60 by 28 to obtain a factor of 2.14; therefore, 2.14 g of urea is equivalent to 1.0 g of urea nitrogen. If the urea nitrogen value of 15 mg/dL is multiplied by 2.14, one obtains a concentration of urea of 32 mg/dL. To convert the urea concentration of 32 mg/dL into mmol/L, one multiplies 32 by 10 and then divides by the molecular weight of urea (i.e., 60) to obtain 5.3 mmol/L.
Rationale: This would not necessarily explain the discrepancy.
B. Wider dynamic range with the automated laboratory analyzer.
Rationale: This is an advantage but would not explain the discrepancy.
C. Lower coefficient of variation with the automated laboratory analyzer.
Rationale: This would result in improved precision but would not explain the discrepancy.
D. Different methods are used for this analysis on these two instruments.
Rationale: Indirect versus direct measurement methods are used on the two different instruments.
E. Greater accuracy using the automated laboratory analyzer.
Rationale: This is not definitive and would not necessarily explain the discrepancy.
B. Increased intact PTH, increased calcium.
C. Decreased intact PTH, decreased calcium.
D. Increased intact PTH, decreased calcium.
E. Increased intact PTH, calcium within the normal range.
Rationale: The circulating intact PTH and serum calcium levels should be low in primary hypoparathyroidism.
B. Increased intact PTH, increased calcium.
C. Decreased intact PTH, decreased calcium.
D. Increased intact PTH, decreased calcium.
E. Increased intact PTH, calcium within the normal range.
Rationale: The circulating intact PTH level is usually low in malignant conditions. Serum calcium is expected to be high in malignant conditions.
B. Increased intact PTH, increased calcium.
C. Decreased intact PTH, decreased calcium.
D. Increased intact PTH, decreased calcium.
E. Decreased intact PTH, calcium within the normal range.
Rationale: The circulating intact PTH level and the serum calcium level should be elevated in primary hyperparathyroidism.
B. Increased intact PTH, increased calcium.
C. Decreased intact PTH, decreased calcium.
D. Increased intact PTH, decreased calcium.
E. Decreased intact PTH, calcium within the normal range.
Rationale: The circulating intact PTH level should be elevated in secondary hyperparathyroidism resulting from renal failure. Serum calcium levels are usually low in secondary hyperparathyroidism caused by renal failure.
Rationale: Because carboxyhemoglobin contains bound carbon monoxide, it is nonfunctional; however, deoxyhemoglobin is functional with regard to oxygen-binding capacity.
B. Deoxyhemoglobin and methemoglobin.
Rationale: Because methemoglobin contains iron in the ferric state, it is nonfunctional; however, deoxyhemoglobin is functional with regard to oxygen-binding capacity.
C. Sickle hemoglobin and methemoglobin.
D. Sickle hemoglobin and deoxyhemoglobin.
Rationale: The sickle cell hemoglobin level (HbS) is not relevant in these contexts.
E. Carboxyhemoglobin and methemoglobin.
Rationale: These two components correspond to nonfunctional forms of hemoglobin, which comprises the difference between So2 and Fo2.
Rationale: Serum levels of phosphate are low in primary hyperparathyroidism.
B. Elevated levels of serum calcium and inorganic phosphate, elevated levels of urine calcium, and low levels of urine inorganic phosphate.
Rationale: Serum phosphate levels are low and urine phosphate levels are high in primary hyperparathyroidism.
C. Elevated levels of serum and urine calcium and low levels of serum and urine inorganic phosphate.
Rationale: Urine phosphate levels are high in primary hyperparathyroidism.
D. Elevated levels of serum calcium, low levels of serum inorganic phosphate, low levels of urine calcium, and elevated levels of urine inorganic phosphate.
Rationale: Urine calcium levels are elevated in primary hyperparathyroidism.
E. Elevated levels of serum and urine calcium, low levels of serum inorganic phosphate, and elevated levels of urine inorganic phosphate.
Rationale: Urine phosphate levels and urine calcium levels are high in primary hyperparathyroidism, and serum phosphate levels are low in primary hyperparathyroidism.
Rationale: Potassium is an important cation and contributes to plasma osmolality.
B. Plasma sodium.
Rationale: Plasma sodium contributes greatly to osmolality; this factor alone can be used to estimate osmolality in plasma.
C. Urine sodium.
Rationale: Urine sodium is important in the differential diagnosis, helping to distinguish between various causes of hyponatremia.
D. Plasma calcium.
Rationale: Calcium does not contribute greatly to plasma osmolality.
E. Plasma glucose.
Rationale: Glucose is an important analyte that contributes to plasma osmolality.
Rationale: Elevated circulating chloride and glucose concentrations will not affect the indirect ISE assay.
B. Elevated circulating protein and lipid levels.
Rationale: Elevated circulating protein and lipid concentrations will lower sodium levels obtained by an indirect method.
C. Decreased circulating protein and lipid levels.
Rationale: Low circulating protein and lipid concentrations will not affect the indirect ISE assay.
D. Decreased circulating protein and glucose levels.
Rationale: Low circulating protein and glucose concentrations will not affect the indirect ISE assay.
E. Decreased circulating protein and chloride levels.
Rationale: Low circulating protein and chloride concentrations will not affect the indirect ISE assay.
Rationale: Prerenal azotemia will be observed in patients with congestive heart failure, resulting in a high BUN/creatinine ratio.
B. A high-protein diet.
Rationale: A high-protein diet will result in a high BUN/creatinine ratio.
C. Acute tubular necrosis.
Rationale: Acute tubular necrosis will result in increases in both BUN and creatinine, with variable effects on the BUN/creatinine ratio.
D. Acute glomerular injury.
Rationale: Acute glomerular injury leads to increases in both BUN and creatinine, resulting in either a normal or elevated BUN/creatinine ratio.
E. Severe liver disease.
Rationale: Liver disease will result in a low BUN/creatinine ratio. Malnutrition will lead to the same effect.
Rationale: Urine creatinine concentrations are not used to calculate the GFR using the MDRD equation.
B. The calculation requires the serum creatinine and BUN levels along with patient age, gender, and race.
Rationale: BUN concentrations are not used to calculate the GFR using the MDRD equation.
C. The calculation requires the serum creatinine and cystatin C levels along with patient age, gender, and race.
Rationale: Cystatin C concentrations are not used to calculate the GFR using the MDRD equation.
D. The calculation requires the serum and urine creatinine levels along with the volume of urine.
Rationale: Urine volume is not used to calculate the GFR using the MDRD equation.
E. The calculation uses the serum creatinine level along with patient age, gender, and race.
Rationale: The estimated GFR is calculated based on the serum creatinine level, age, gender, and race of the patient.
Rationale: The patient has a metabolic acidosis with an elevated anion gap.
B. Metabolic acidosis with a normal anion gap.
C. Respiratory acidosis with an elevated anion gap.
Rationale: The Pco2 is elevated in respiratory acidosis.
D. Renal tubular acidosis (RTA).
Rationale: In renal tubular acidosis, the anion gap is normal.
E. Respiratory acidosis with a normal anion gap.
Rationale for B and E: In this patient, the anion gap is elevated.
B. Respiratory acidosis with an elevated anion gap.
C. Metabolic acidosis with a normal anion gap.
Rationale: In the setting of diarrhea, the metabolic acidosis occurs as the result of an increased loss of HCO3− through the gastrointestinal tract. The anion gap is normal in diarrhea.
D. Metabolic acidosis with an elevated anion gap.
Rationale for A, B, and D: The anion gap is normal in diarrhea.
E. RTA type 2 with an elevated anion gap.
Rationale: RTA type 2 is caused by decreased reabsorption of HCO3−. The anion gap is normal in renal tubular acidosis.
Rationale: The pH is low in metabolic acidosis.
B. Respiratory acidosis.
Rationale: The pH is low in respiratory acidosis.
C. Metabolic alkalosis.
Rationale: The pH is low in metabolic alkalosis. Pco2 is normal or slightly increased.
D. Diabetic ketoacidosis (DKA).
Rationale: The pH is low in DKA.
E. Respiratory alkalosis.
Rationale: The Pco2 is low in respiratory alkalosis.
Rationale: The pH is elevated in metabolic alkalosis, but it is near normal in this patient because of respiratory compensation (increased Pco2).
B. Metabolic alkalosis.
Rationale: The pH is elevated in metabolic alkalosis.
C. Metabolic acidosis.
Rationale: The pH is low in metabolic acidosis.
D. Respiratory acidosis.
Rationale: The pH is low in respiratory acidosis.
E. Respiratory alkalosis with metabolic compensation.
Rationale: The Pco2 is not elevated in respiratory alkalosis.
Rationale: Microalbuminuria is not used to predict glomerulonephritis.
B. Contributes to multiple myeloma staging.
Rationale: Beta-2 microglobulin is used in the staging of multiple myeloma.
C. Identifies the presence of end-stage renal disease.
Rationale: Microalbuminuria is not used to detect end-stage renal disease.
D. Predicts kidney transplant rejection.
Rationale: Microalbuminuria is not used to predict kidney rejection.
E. Predicts diabetic nephropathy.
Rationale: Microalbuminuria is an indicator of deteriorating renal function in diabetic patients and is the first abnormal biochemical marker to appear in diabetic nephropathy.
Rationale: BUN and creatinine are elevated in nephrotic syndrome, but they are not the major abnormalities in this setting.
B. Increased serum cystatin C concentration.
Rationale: Cystatin C is elevated in nephrotic syndrome but is not the major abnormality in this setting.
C. Increased serum cholesterol concentration.
Rationale: Cholesterol is elevated in nephrotic syndrome but is not the major abnormality in this setting.
D. Microalbuminuria.
Rationale: Significant proteinuria, much greater than that seen with microalbuminuria, is observed in nephrotic syndrome.
E. Proteinuria of greater than 3.0 g/day.
Rationale: Significant proteinuria is observed in the nephrotic syndrome.
Rationale: Renal artery ultrasound is used when prerenal defects, such as would be implied by a BUN/creatinine ratio more than 20, are suspected.
B. Serum and urine osmolality.
Rationale: The most likely etiology of his renal failure is intrinsic renal disease because the patient’s BUN/creatinine ratio is between 10 and 20, and he was recently exposed to nephrotoxic agents. To determine whether the damage is in the glomerular or tubular compartment of the kidney, examination of urine and serum osmolality during fluid restriction is informative. A normal ratio of urine to serum osmolality implies properly functioning tubules and suggests glomerular injury. A low ratio (< 1.2 in a fluid-restricted sample) suggests the primary concentration mechanism localized in the tubules is defective.
C. Serum potassium.
Rationale: Serum potassium is elevated in acute renal failure but is not helpful in etiologic determinations.
D. Plasma aminoglycoside levels.
Rationale: Aminoglycoside levels may be informative after the presence of tubular injury is confirmed.
E. Renal biopsy.
Rationale: An invasive procedure such as a renal biopsy is not indicated at this time.
Rationale: This is a typical profile seen in hypoalbuminemic patients.
B. 6 g/dL, 0.89 g/dL, 6 g/dL.
Rationale: The classic finding in renal failure of tubular etiology is a low calcium level with a high phosphate level. In renal tubular disease, phosphate excretion is prevented because of nonresponsiveness to PTH. This hyperphosphatemia, along with defective vitamin D regulation, contributes to hypocalcemia. The ionized calcium is a reflection of the biologically available calcium and should also be low.
C. 9 g/dL, 0.89 g/dL, 6 g/dL.
Rationale: Total calcium, not just the ionized fraction, should be low in renal failure.
D. 9 g/dL, 0.99 g/dL, 4 g/dL.
Rationale: This is a normal profile.
E. 6 g/dL, 0.99 g/dL, 2 g/dL.
Rationale: This profile is not consistent with a renal tubular etiology.
B. Metabolic acidosis with an elevated anion gap.
Rationale: In metabolic acidosis, the Pco2 is normal.
C. Respiratory acidosis with an elevated anion gap.
Rationale: In respiratory alkalosis, the anion gap is normal.
D. RTA with an elevated anion gap.
Rationale: In RTA, the anion gap is normal.
E. Respiratory acidosis with a normal anion gap.
Rationale: An elevated Pco2 level characterizes all causes of respiratory acidosis.
Rationale: The pH is low in metabolic acidosis.
B. Respiratory acidosis.
Rationale: The pH is low in respiratory acidosis.
C. Metabolic alkalosis.
Rationale: The Pco2 is normal in metabolic alkalosis.
D. Respiratory alkalosis.
Rationale: Respiratory alkalosis is caused by hyperventilation, which decreases the Pco2.
E. DKA.
Rationale: The pH is low in DKA.
B. Renal regulation of HCO3−.
Rationale: Renal compensation in respiratory alkalosis occurs when the kidneys excrete increased amounts of HCO3−.
C. Hyperventilation.
Rationale: Hyperventilation would decrease the Pco2 and increase the pH.
D. Renal excretion of H+.
Rationale: Excretion of hydrogen ions would result in an increase in pH.
E. Renal excretion of organic acids.
Rationale: Excretion of organic acids occurs in metabolic acidosis, resulting in an increase in pH.
Rationale: Serum PTH levels are elevated in rickets.
B. Low levels of calcium, 25-hydroxyvitamin D, and inorganic phosphate, and an elevated PTH level.
Rationale: Rickets is a childhood disease that is primarily caused by vitamin D deficiency.
C. Low levels of calcium and 25-hydroxyvitamin D, and elevated levels of inorganic phosphate and PTH.
Rationale: Serum phosphate levels are low in rickets.
D. Low levels of calcium, 25-hydroxyvitamin D, and PTH, and an elevated inorganic phosphate level.
Rationale: Serum PTH levels are elevated and serum phosphate levels are low in rickets.
E. Elevated levels of calcium and PTH and low levels of 25-hydroxyvitamin D and inorganic phosphate.
Rationale: Serum calcium levels are low in rickets.
Rationale: Oxyhemoglobin is functional, but carboxyhemoglobin is bound to carbon monoxide and, therefore, is nonfunctional and cannot bind oxygen or carbon dioxide.
B. Oxyhemoglobin and deoxyhemoglobin.
Rationale: Both oxyhemoglobin and deoxyhemoglobin are functional.
C. Oxyhemoglobin and methemoglobin.
Rationale: Oxyhemoglobin is functional, but methemoglobin contains the ferric form of iron; therefore, it is not functional and cannot bind oxygen or carbon dioxide.
D. Deoxyhemoglobin and carboxyhemoglobin.
Rationale: Deoxyhemoglobin is able to bind to oxygen, but carboxyhemoglobin is bound to carbon monoxide; therefore, it is nonfunctional and cannot bind oxygen or carbon dioxide.
E. Deoxyhemoglobin and methemoglobin.
Rationale: Deoxyhemoglobin can readily accept oxygen, but methemoglobin contains the ferric form of iron; therefore, it is not functional and cannot bind oxygen or carbon dioxide.
Rationale: Contamination with potassium-ethylenediaminetetraacetic acid (EDTA) will cause spurious hyperkalemia and hypocalcemia.
B. Chronic kidney failure.
Rationale: Chronic kidney failure is commonly associated with hyperkalemia, hyperphosphatemia, and hypocalcemia. This is unlikely in this patient with normal creatinine and normal phosphatemia.
C. Renal tubular acidosis.
Rationale: RTA will cause hyperchloremic metabolic acidosis with low total CO2. Proximal RTA can cause hypophosphatemia, whereas distal RTA typically is associated with hypokalemia and hypercalcemia.
D. Primary hyperaldosteronism.
Rationale: Excessive mineralocorticoid activity results in hypokalemia with metabolic alkalosis.
E. Congenital adrenal hyperplasia.
Rationale: Mineralocorticoid deficiency can cause hyperkalemia with metabolic acidosis. Calcium is usually normal.
Rationale: Hypoadrenalism leads to hypotension, hyperkalemia, and metabolic acidosis with decreased bicarbonate and elevated chloride.
B. Conn syndrome.
Rationale: Hyperaldosteronism causes renal loss of potassium and protons, resulting in hypokalemia and hypochloremic metabolic alkalosis.
C. Syndrome of inappropriate antidiuretic hormone secretion.
Rationale: Excessive secretion of ADH leads to increased water reabsorption and hyponatremia.
D. Diabetes insipidus.
Rationale: Lack of ADH or poor response to ADH leads to loss of water with resulting polydipsia and hypernatremia if water intake is restricted.
E. Diabetes mellitus.
Rationale: Osmotic diuresis caused by hyperglycemia can lead to dehydration and hypernatremia if water intake is restricted.
Rationale: This would result in increased cortisol.
B. Lung cancer–producing ADH.
Rationale: ADH is high-normal but other results (hypernatremia) are inconsistent with excess ADH production.
C. Nephrogenic diabetes insipidus.
Rationale: Hypercalcemia can cause nephrogenic diabetes insipidus; ADH is normal or elevated in the presence of water losses.
D. Central diabetes insipidus.
Rationale: This condition results in decreased ADH.
E. Diabetic nephropathy.
Rationale: This condition causes proteinuria and progressive decline in the GFR; water reabsorption is usually preserved.
Rationale: This would indicate adrenal insufficiency.
B. Increased urine osmolality after water deprivation and ADH administration.
Rationale: In central diabetes insipidus, urine remains dilute with water deprivation but normalizes after ADH administration, while in nephrogenic diabetes insipidus urine osmolality does not respond to ADH.
C. Elevated 1,25-dihydroxyvitamin D.
Rationale: Macrophages in granulomatous tissue, such as in sarcoidosis, have high levels of CYP27B1 enzyme (25-hydroxyvitamin D-1α-hydroxylase), which converts 25-hydroxyvitamin D to 1,25-dihydroxyvitamin D, causing hypercalcemia.
D. Elevated PTH-related peptide (PTHrP).
Rationale: Increased PTHrP is a possible cause of malignancy-associated hypercalcemia; in this case, sarcoidosis with elevated 1,25-dihydroxyvitamin D is a more likely cause of hypercalcemia. Rarely, sarcoidosis has been associated with elevated PTHrP; however, in most cases, it is undetectable.
E. Elevated C-peptide after a standard meal.
Rationale: This is a rarely used test to help distinguish type 1 from type 2 diabetes.
Rationale: Hyperviscosity due to leukocytosis is usually not seen with blast counts less than 100,000/μL. Rhabdomyolysis is not typically associated with chronic myelogenous leukemia and would more likely result in hyperkalemia due to cell lysis.
B. Adrenal insufficiency due to leukemic infiltration.
Rationale: This would likely result in hyperkalemia with metabolic acidosis.
C. Acute tubular necrosis of the kidney.
Rationale: Unlikely with normal creatinine and hypokalemia.
D. Pseudohypokalemia due to leukocytosis.
Rationale: Low glucose and potassium levels are due to the consumption of glucose and potassium by metabolically active leukocytes before serum separation.
E. Lactic acidosis due to leukemic anaerobic metabolism.
Rationale: This is a rare complication of acute leukemia, possibly due to anaerobic metabolism by leukemic cells, and would manifest as an increased anion gap acidosis with low total CO2, often with hypoglycemia and normal or elevated potassium.
Rationale: With adequate water intake in the setting of severe hyperglycemia with osmotic diuresis, the BUN/creatinine ratio can be normal or low despite severe water and electrolyte losses and intracellular dehydration.
B. The hyponatremia is probably due to increased extracellular water content.
Rationale: Hyperglycemia causes osmotic expansion of the extravascular compartment and dilutional hyponatremia. Plasma sodium is corrected according to the following formula: Na (corrected) = Na (measured) + 0.016 × (glucose – 100), which gives a sodium level of 147 mmol/L in the absence of osmotic expansion.
C. The hyperkalemia indicates excess total body potassium.
Rationale: Although patients can present with hyperkalemia, mainly resulting from mild acidosis and decreased activity of the sodium-potassium-ATPase pump, leading to intracellular to extracellular shifts, patients with hyperosmolar hyperglycemia are truly potassium depleted as the result of osmotic diuresis and renal losses. Careful replenishment of potassium guided by plasma levels is critical to avoid arrhythmias, cardiac arrest, and respiratory muscle weakness.
D. There is significant ketoacidosis due to insulin deficiency.
Rationale: Typically, DKA is accompanied by severe metabolic acidosis characterized by pH less than 7.30 and bicarbonate less than 15 mmol/L.
E. Effective plasma osmolality is consistent with hyperosmolar hyperglycemic state.
Rationale: Plasma osmolality can be calculated by the following formula: 2 × Na + glucose/18 + BUN/2.8, giving a result of 329 mOsm/kg in this patient (reference range, 285 to 295). Effective osmolality does not include BUN as urea freely crosses cell membranes. This patient’s effective osmolality is 323 mOsm/kg, consistent with a hyperglycemic hyperosmolar state (> 320 mOsm/kg according to American Diabetic Association guidelines).
Rationale: This presentation mimics DKA, but the lack of elevated hemoglobin A1c and autoantibodies associated with type 1 diabetes makes it less likely.
B. Fatty acid oxidation defect.
Rationale: Typically presents with hypoglycemia and low ketones.
C. Pyruvate dehydrogenase deficiency.
Rationale: Typically presents with lactic acidosis and elevated pyruvate.
D. Malnutrition with chronic vitamin B12 deficiency.
Rationale: Unlikely in the presence of normal levels of vitamin B12 and homocysteine.
E. Adenosylcobalamin synthesis defect.
Rationale: This condition causes accumulation of methylmalonic acid but not homocysteine, explaining the patient’s condition.
Rationale: Release of intracellular phosphates from organic foods often results in increases in phosphatemia by 15% approximately 2 hours after a standard meal. Within 2 to 3 hours postprandial, insulin induces glucose and phosphate uptake by cells with gradual normalization of phosphate levels.
B. A shift from supine to upright position causes increases in plasma calcium.
Rationale: Increased total calcium parallels a 10% to 15% increase in albumin.
C. Acute ethanol intoxication is frequently associated with metabolic alkalosis.
Rationale: Metabolism of ethanol to acetate leads to metabolic acidosis, often compounded by associated ketoacidosis resulting from inhibition of glycolysis by alcohol.
D. Vigorous exercise often leads to lower potassium levels.
Rationale: Vigorous exercise can result in transient hyperkalemia, as high as 8 mmol/L in arterial blood, especially in poorly trained individuals. Well-trained athletes have a higher density of sodium-potassium exchangers in their muscles and much lower increases in potassium.
E. Stress (e.g., during acute myocardial infarction) often causes hyperkalemia.
Rationale: Catecholamines acting through β-adrenergic receptors induce intracellular influx and renal excretion of potassium. Hypokalemia is frequent in acute myocardial infarction and can predispose patients to severe arrhythmias.
Rationale: This will cause hemolysis and hyperkalemia in both plasma and serum.
B. Prolonged tourniquet time with fist clenching.
Rationale: This will cause release of potassium from muscle; however, both plasma and serum potassium should be elevated.
C. Thrombocytosis.
Rationale: Elevated platelet counts will cause release of potassium during clotting, with serum potassium higher than plasma (about 0.15 mmol/L per 100,000 platelets/μL).
D. Leukocytosis.
Rationale: Leukocytosis can cause mild pseudohyperkalemia in both serum and plasma samples caused by the release from leukocytes, particularly from fragile cells such as in chronic leukemias.
E. Familial pseudohyperkalemia.
Rationale: This condition is characterized by plasma potassium higher than serum and increases in plasma and serum potassium if cell separation is delayed.
Rationale: Congenital defects in organic acid metabolism can cause metabolic acidosis with increased anion gap.
B. Type 1 (distal) RTA.
Rationale: Characterized by failure to acidify urine (pH > 5.3, usually ~ 6.5) in the presence of hyperchloremic metabolic acidosis.
C. Type 2 (proximal) RTA.
Rationale: This condition best explains hyperchloremic metabolic acidosis with proper urine acidification, glycosuria, and hypophosphatemia.
D. Hyporeninemic hypoaldosteronism.
Rationale: This condition (type IV renal tubular acidosis) would cause hyperkalemia and would not explain glycosuria and hypophosphatemia.?
E. DKA.
Rationale: Ketoacidosis would cause increased anion gap metabolic acidosis with hyperglycemia.
Rationale: This would be expected with organic aciduria due to defects in methylmalonic acid metabolism.
B. Increased urinary pH after NH4Cl load.
Rationale: Failure to acidify urine after acid load is typical of type 1 RTA.
C. Elevated fractionary urinary excretion of HCO3 after bicarbonate load.
Rationale: Losses of bicarbonate in the proximal renal tubule are characteristic of type 2 RTA.
D. Decreased plasma aldosterone/renin ratio.
Rationale: In hyporeninemic hypoaldosteronism the ratio may be normal, increased, or decreased, but both aldosterone and renin are low.
E. High plasma levels of β-hydroxybutyrate.
Rationale: This is one of the ketone bodies elevated in DKA.
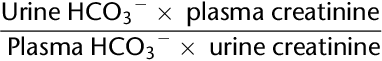
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


Basicmedical Key
Fastest Basicmedical Insight Engine