Chapter 8 Alex J. Rai; Michael A. Pesce; Tilla S. Worgall A. Biopsy with subsequent staging/grading. B. Percent free PSA. C. Imaging studies to determine prostate volume and density. D. Digital rectal exam (DRE). E. No further action. 2. A patient has recently been diagnosed with ovarian cancer and will be starting a cisplatin-based chemotherapy regimen. Which one of the following is the best serum tumor marker to determine whether her treatment is effective? A. Carcinoembryonic antigen (CEA). B. Carbohydrate antigen 19-9 (CA-19-9). C. Cancer antigen 125 (CA-125). D. Alpha fetoprotein (AFP). E. Human chorionic gonadotropin (hCG) subunits. 3. Which one of the following tumor markers is approved by the Food and Drug Administration (FDA) for screening purposes? A. PSA with DRE for prostate cancer. B. CA-125 and ultrasound imaging for breast cancer. C. CEA for lung cancer in a patient with a history of smoking. D. AFP for hepatocellular carcinoma (HCC) in a patient with elevated levels of aspartate and alanine aminotransferase. E. Serial hCG measurements for breast cancer. 4. Which one of the following best describes the biological active forms of the natriuretic peptides that are found in serum? A. Brain natriuretic peptide (BNP) and N-terminal-prohormone of BNP (NT-ProBNP). B. BNP and pre-prohormone of BNP. C. Atrial natriuretic peptide (ANP) and BNP. D. Urodilatin and BNP. E. ANP and NT-prohormone of BNP. 5. Which one of the following best describes the clinical utility of BNP and NT-proBNP? A. Elevated BNP or NT-proBNP levels can be used to detect an acute myocardial infarction. B. Elevated BNP or NT-proBNP levels can be used to detect unstable angina. C. Elevated BNP or NT-proBNP levels can be used to detect cardiac ischemia. D. Elevated BNP or NT-proBNP levels can be used to screen for congestive heart failure in asymptomatic individuals. E. Normal BNP or NT-proBNP levels can be used to rule out congestive heart failure. 6. Which one of the following explains why creatine kinase (CK)-MB can be used to detect myocardial necrosis? A. CK-MB is detected in the blood before myoglobin after acute myocardial infarction. B. CK-MB is found only in cardiac muscle. C. CK-MB is detected in blood before troponin I or troponin T after an acute myocardial infarction. D. CK-MB is known to rise and fall predictably after an acute myocardial infarction. E. CK-MB is a better predictor than troponin I or T for risk stratification of patients with acute myocardial infarctions. 7. Which one of the following best describes the structure of CK-MB isoforms (i.e., CK-MB1 and CK-MB2)? A. Macromolecular complexes consisting of CK-MB bound to IgG. B. CK mitochondrial complexes. C. Artifactual forms of CK caused by binding of CK to albumin. D. Posttranslational modification of CK-MB. E. CK complexes consisting of CK-MB bound to immunoglobulin A (IgA). 8. Which one of the following serum proteins is most useful in determining whether an acute-phase response is due to a bacterial infection? B. C4. C. Albumin. D. C-reactive protein (CRP). E. Transferrin. 9. Which one of the following is consistent with the European Society of Cardiology–American College of Cardiology established criteria for detection of an acute myocardial infarction? A. Measurement of troponin, myoglobin, and CK-MB. B. Measurement of CK-MB and troponin. C. Measurement of myoglobin and troponin. D. Measurement of the rise and fall of troponin. E. Measurement of CK-MB isoforms and troponin. 10. Which one of the following tests can be used as an early predictor of coronary artery disease in asymptomatic individuals? B. Ischemia modified albumin. C. High-sensitivity CRP (hsCRP). D. BNP. E. CK-MB. 11. Which one of the following combinations of biomarkers best detects cardiac ischemia? A. Ischemia-modified albumin (IMA) and glycogen phosphorylase isoenzyme BB. B. IMA and troponin. C. IMA and free fatty acid binding protein. D. IMA and BNP. E. IMA and myosin light chain. 12. Serum levels of lactate dehydrogenase (LDH) isoenzymes and haptoglobin were measured in a patient with liver disease. The LDH-1 fraction was greater than the LDH-2 fraction and haptoglobin was undetectable. Which one of the following interpretations is compatible with these laboratory results? A. Concurrent cardiac ischemia. B. Concurrent congestive heart failure. C. Concurrent unstable angina. D. Artifact due to hemolysis. E. Increasing liver dysfunction. 13. Which one of the following reasons best explains how serum myoglobin levels can be used as a cardiac marker for patients admitted to the emergency department 4 hours after the onset of chest pain? A. Elevated circulating myoglobin levels are diagnostic for an acute myocardial infarction. B. Circulating myoglobin levels are elevated for days after an acute myocardial infarction. C. Circulating myoglobin levels become elevated after troponin I or troponin T after an acute myocardial infarction. D. Circulating myoglobin levels peak after CK-MB levels rise after an acute myocardial infarction. E. Normal circulating myoglobin levels can be used to rule out an acute myocardial infarction. 14. The troponin complex is composed of which one of the following components? A. Troponin T, calcium, and actin. B. Troponin C, myoglobin, and actin. C. Troponin I, actin, and tropomyosin. D. Troponin I, troponin T, and troponin C. E. Troponin T, tropomyosin, and calcium. 15. An external proficiency test survey sample to evaluate troponin I was sent to laboratories across the United States. The results of this interlaboratory survey for this sample showed up to a tenfold difference in troponin I levels among the laboratories. Which one of the following reasons represents the most likely cause of this variability? A. Some troponin I methods are not accurate or precise. B. Some troponin I methods cross-react with troponin T. C. Some troponin I methods are standardized. D. Some troponin I methods cross-react with troponin C. E. Some troponin I methods measure different molecular forms of troponin I. 16. Which one of the following best describes the major advantage of using either troponin I or troponin T, rather than CK-MB, for detecting an acute myocardial infarction? B. The troponin complex is not found in the cytosolic pool. C. Troponin is released early into blood after an acute myocardial infarction because of degradation of the contractile apparatus. D. Troponin is part of the thick filament (myosin) of muscle. E. Cardiac troponin T and I subunits are distinctly different from their skeletal muscle counterparts. 17. Troponin I is used to evaluate infants with cardiac disease. Which one of the following best explains why troponin T cannot be used to evaluate cardiac injury in infants? A. Bilirubin interferes with the troponin T method. B. Skeletal muscle troponin T found in newborns cross-reacts with troponin T. C. Precision of the troponin T method in newborns has not been established. D. The troponin T method requires a large sample volume and is not suitable for use in newborn testing. E. The analytical sensitivity of the troponin T method is not high enough and therefore cannot be used for newborn testing. 18. Troponin T and troponin I are the most frequently used biomarkers for the detection of an acute myocardial infarction. Although the measured troponin I results from different hospitals can be significantly different, there is usually no significant difference in troponin T results obtained from different hospitals. Which one of the following best explains the low interlaboratory variability for troponin T? B. Troponin T is more stable in blood than troponin I. C. The troponin T method is more precise than the troponin I method. D. Only one method is available to measure troponin T. E. Only one molecular form of troponin T is found in blood. 19. A 23-year-old pregnant woman, near the end of her first trimester, has a quantitative pregnancy test performed. Surprisingly, her hCG is 350 IU/mL, which is a value much less than would be expected based on her gestational age. Which one of the following is the best explanation for a falsely low serum level of hCG? A. Heterophilic antibodies in the sample. B. Imprecision of the assay. C. Calibration using a sigmoidal curve. D. Multiplex enzyme-linked immunosorbent assay (ELISA) used to perform this assay. E. Presence of the hook effect. 20. A 70-year-old man presents to his physician with generalized bone pain, a recently broken hip, weight loss, nausea, and constipation. Which one of the following serum protein electrophoresis test results would be diagnostic of multiple myeloma? A. Presence of a band in the beta globulin fraction at a concentration of 0.2 g/dL. B. Presence of a band in the gamma globulin fraction at a concentration of 3 g/dL. C. Decreased level of a band in the albumin fraction to a concentration of 0.2 g/dL. D. Increased levels of bands in the alpha-1 and alpha-2 globulin fractions at a concentration of 0.2 g/dL each. E. Presence of a band in the prealbumin fraction at a concentration of 0.5 g/dL. 21. Which one of the following serum protein electrophoresis patterns best suggests an acute inflammatory response? A. Increased albumin; increased alpha-1, alpha-2, and beta globulins; and normal gamma globulins. B. Decreased albumin, increased alpha-1 and alpha-2 globulins, and normal beta and gamma globulins. C. Decreased albumin, decreased alpha-1 and alpha-2 globulins, and normal beta and gamma globulins. D. Increased albumin, decreased alpha-1 and alpha-2 globulins, normal beta globulins, and increased gamma globulins. E. Normal albumin; increased alpha-1, alpha-2, and beta globulins; and normal gamma globulins. 22. AFP is a major embryonic protein. After 12 weeks of gestation, which one of the following statements best describes the predominant site(s) of AFP synthesis? B. The fetal pancreas. C. The yolk sac and fetal liver. D. The fetal liver. E. The fetal kidney. 23. The serum protein and immunofixation electrophoresis (IFE) patterns shown in Figure 8-1and Table 8-1 were determined for a 55-year-old man with type 1 diabetes. The protein fractions are shown below. Which one of the following represents the best interpretation of these protein patterns? A. This is the serum protein electrophoresis pattern that is seen in severe liver disease. B. This is the serum IFE pattern for a monoclonal protein. C. This is the serum protein electrophoresis pattern that is usually seen in nephrosis. D. This is the serum protein electrophoresis pattern that is seen in iron deficiency anemia. E. This is the serum protein electrophoresis pattern that is seen in alpha-1 antitrypsin deficiency. Table 8-1 Protein Fractions Determined by Serum Protein Electrophoresis You have identified a biomarker signature composed of three proteins in plasma that appears to be useful in the management of patients with prostate cancer. This signature is found in virtually 100% of this population. Use this scenario to answer the following five questions. 24a. Which one of the following performance characteristics for this test is high? B. Specificity. C. Precision. D. Negative predictive value. E. Accuracy. 24b. The biomarker signature is found only in prostate cancer patients and is not detected in other patient populations, such as patients with other types of cancer, or in healthy volunteers, Which one of the following performance characteristics is high? B. Specificity. C. Positive predictive value. D. Negative predictive value E. Accuracy. 24c. Assume that this new biomarker signature has a low specificity and, therefore, is not useful for population-based screening for prostate cancer. Which one of the following performance characteristics can be improved by using it only in a high-risk clinic setting that serves only patients with a family history of this disease? B. Precision. C. Positive predictive value. D. Reference interval. E. Accuracy. 24d. This novel biomarker signature is not detectable in a cohort of patients who are free of prostate cancer. The inability to detect this signature in such a population would produce a high value for which one of the following characteristics? B. Precision. C. Positive predictive value. D. Negative predictive value. E. Accuracy. 24e. This newly discovered biomarker signature correlates well with the existing gold standard methodology, which is immunohistochemical analysis of prostate biopsy tissue. Which one of the following performance characteristics is high? B. Specificity. C. Positive predictive value. D. Negative predictive value. E. Accuracy. 25. Serum protein and IFE patterns were obtained for a 65-year-old man with a chronic history of fatigue and back pain. Protein fractions are shown in Figure 8-2 and Table 8-2. Which one of the following represents the best interpretation of these patterns? B. SPEP showed low albumin and slightly elevated gamma globulin levels and the possibility of a monoclonal protein in the gamma globulin region, which was identified by IFE as a free lambda light chain. C. SPEP showed low albumin and slightly elevated polyclonal gamma globulin levels. No monoclonal protein was detected by IFE. D. SPEP showed low albumin and slightly elevated gamma globulin levels and the possibility of a monoclonal protein in the gamma globulin region. No monoclonal protein was detected by IFE. The narrow band in the SPEP pattern is probably due to fibrinogen. E. SPEP showed low albumin and slightly elevated gamma globulin levels and the possibility of a monoclonal protein in the gamma globulin region, which was identified by IFE as a monoclonal IgA lambda protein. Table 8-2 Protein Fractions Determined by Serum Protein Electrophoresis The epidermal growth factor receptor (EGFR) serves an important role in the transmission of growth signals from the plasma membrane to the nucleus. In recent years, multiple somatic mutations in EGFR have been characterized in tumor tissue derived from patients with non–small cell lung cancer (NSCLC). Use this scenario to answer the following four questions. 26a. Which one of the following best describes the significance of the L858R mutation in EGFR for patients with NSCLC? A. Acquisition of this mutation marks the transition from low-grade to high-grade cancer. B. Acquisition of this mutation marks the transition from early-stage to late-stage disease. C. This mutation correlates with sensitivity to chemotherapy using tyrosine kinase inhibitors. D. This mutation correlates with resistance to chemotherapy using tyrosine kinase inhibitors. E. This mutation portends a favorable outcome (i.e., is an indicator of good prognosis). 26b. Which one of the following best describes the significance of the T790M mutation in EGFR? A. Acquisition of this mutation marks the transition from low-grade to high-grade cancer. B. Acquisition of this mutation marks the transition from early-stage to late-stage disease. C. This mutation correlates with sensitivity to chemotherapy using tyrosine kinase inhibitors. D. This mutation correlates with resistance to chemotherapy using tyrosine kinase inhibitors. E. This mutation portends a favorable outcome (i.e., is an indicator of good prognosis). 26c. Which one of the following is the best serological tumor marker to monitor treatment for this patient with lung cancer? B. PSA. C. AFP. D. hCG. E. CEA. 26d. The assay to measure CEA levels in blood is not useful for population-based cancer screening. Which one of the following answers best describes why this is true? B. CEA has poor specificity for lung cancer; there are multiple nonmalignant conditions resulting in elevated CEA levels. C. The dynamic range of this assay is limited and not suitable for patients with late-stage or metastatic disease. D. The analytical sensitivity of this assay does not meet the needs for early detection. E. The assay is imprecise (i.e., the coefficient of variation is > 20%). 27. Which one of the following statements best describes the setting(s) in which maternal serum measurements of AFP are most useful? A. Anencephaly and myelomeningocele. B. Anencephaly, myelomeningocele, and closed spina bifida. C. Anencephaly and closed spina bifida. D. Trisomy 21. E. Trisomy 18. 28. Which one of the following pairs consists only of “negative” acute-phase proteins? A. Albumin and alpha-1-antitrypsin. B. Albumin and alpha-2-macroglobulin. C. Haptoglobin and CRP. D. Albumin and transferrin. E. Transferrin and C3. 29. Which one of the following represents the currently recommended optimal approach for first-trimester maternal screening for the presence of Down syndrome? A. Nuchal translucency (NT), free beta-hCG, and AFP. B. NT, free beta-hCG, and pregnancy-associated plasma protein A (PAPP-A). C. NT, free beta-hCG, and inhibin A. D. NT, free beta-hCG, and unconjugated estriol (uE3). E. NT, PAPP-A, and inhibin A. 30. The prevalence of neural tube defects (NTDs) in the general population of the United States significantly decreased when foods were supplemented with which one of the following nutrients? B. Folic acid. C. Vitamin D. D. Vitamin K. E. Vitamin E. 31. Maternal serum alpha-fetoprotein (MSAFP) levels are used to screen for fetal NTDs in pregnant women. The risk of NTDs for each patient is calculated by using the multiple of the medians (MOM), which is determined by obtaining a median MSAFP value in unaffected pregnancies for each gestational week and dividing the patient’s MSAFP value by the median MSAFP value for the relevant gestational week. The cutoff for predicting a fetal NTD is a value of 2.5 or higher MOM. In addition, MSAFP levels increase with increasing gestational age. Therefore, the correct gestational age is extremely important in calculating the risk of a fetal NTD. If the risk of a fetal NTD is calculated based on the MOM obtained at a gestational age of 19 weeks, but the true gestational age is 15 weeks, which one of the following best explains the risk of a fetal NTD? A. Increased, because the MOM will be higher than expected. B. Increased, because the MOM will be lower than expected. C. Decreased, because the MOM will be lower than expected. D. Decreased, because the MOM will be higher than expected. E. Cannot be determined; therefore, another sample should be drawn to repeat the MSAFP determination. 32. Amniotic fluid alpha-fetoprotein (AFAFP) levels are used to help in the diagnosis of NTDs. The risk of a fetal NTD for each patient is calculated by using the MOM, which is determined by obtaining a median AFP level in unaffected pregnancies for each gestational week and dividing the patient’s AFP value by the median AFP value for the relevant gestational week. The cutoff for diagnosing an NTD is a value of 2.0 or higher MOM. In addition, AFAFP concentrations decrease with increasing gestational age. Therefore, the correct gestational age is extremely important in calculating the risk of NTD. If the risk of a fetal NTD is calculated based on the MOM obtained at a gestational age of 20 weeks, but the true gestational age is 15 weeks, then which one of the following statements best describes the actual risk of a fetal NTD in this case? A. Increased, because the actual MOM is higher than the calculated MOM. B. Increased, because the actual MOM is lower than the calculated MOM. C. Decreased, because the actual MOM is lower than the calculated MOM. D. Decreased, because the MOM is higher than the calculated MOM. E. Slightly decreased, but will not significantly affect the NTD risk calculation. 33. MSAFP levels are used during pregnancy to calculate the risk of fetal NTDs. MSAFP levels are affected by maternal weight. The weight correction for MSAFP levels is performed by comparing the weight of the patient with the weight that is used in a reference population (which is 140 lb) to calculate the risk of NTD. Therefore, which one of the following profiles would a 195-lb woman have if the weight correction was not performed? A. Increased serum MSAFP level and increased risk of NTDs. B. Increased serum MSAFP level and decreased risk of NTDs. C. Decreased serum MSAFP level and increased risk of NTDs. D. Decreased serum MSAFP level and decreased risk of NTDs. E. No change in the risk for NTDs. 34. When a serum sample is placed in an electric field connected to a buffer at a pH of 8.6, which one of the following statements is correct regarding the electrophoretic migration of the proteins? A. Proteins have a positive charge and migrate toward the anode. B. Proteins have a positive charge and migrate toward the cathode. C. Proteins have a negative charge and migrate toward the anode. D. Proteins have a negative charge and migrate toward the cathode. E. Proteins all have the same isoelectric point. 35. Screening for Down syndrome in the second trimester can be performed with the following four serum biomarkers (i.e., the quadratic screen): AFP, uE3, hCG, and inhibin A. Which one of the following patterns is most consistent with a high risk of Down syndrome? A. Elevated AFP, hCG, and inhibin A; low uE3. B. Elevated inhibin A; low AFP, hCG, and uE3. C. Elevated AFP; low or normal uE3, hCG, and inhibin A. D. Elevated inhibin A and uE3; low AFP and hCG. E. Elevated hCG and inhibin A; low AFP and uE3. 36. In the context of maternal serum screening in the second trimester to detect fetal Down syndrome, which one of the following statements provides the best explanation of the advantage of the quadratic screen (i.e., AFP, uE3, hCG, and inhibin A) over the triple screen (i.e., AFP, uE3, and hCG)? A. All cases of fetal Down syndrome are detected. B. There is an increase in the detection rate of fetal Down syndrome. C. The reagent cost is significantly reduced. D. Fetal trisomy 18 cases can be detected. E. The analytical method is automated. 37. Screening for a fetus with Edwards syndrome (i.e., trisomy 18) is performed in the second trimester by measuring maternal serum levels of AFP, uE3, and hCG. Which one of the following patterns best identifies a fetus with trisomy 18? A. AFP is elevated; uE3 and hCG are normal. B. AFP is very elevated; uE3 and hCG are low. C. uE3 and hCG are elevated; AFP is low. D. AFP, uE3, and hCG are all low. E. hCG is elevated; AFP and uE3 are low. You are a clinical laboratory director at a cancer center charged with the selection, design, and validation of new tumor marker assays. Use this scenario to answer the following four questions. 38a. Which one of the following performance characteristics would best serve to implement an assay to measure AFP levels in both the general population and in a population of patients with cancer? B. Analytical sensitivity at the low end. C. High precision. D. Minimal analytical interferences from drugs. E. Standardized results between different assays. 38b. Which one of the following performance characteristics would best serve to implement an assay for detecting recurrent prostate cancer in patients who have undergone radical prostatectomy? B. Analytical sensitivity at the low end. C. High precision. D. Minimal analytical interferences from drugs. E. Standardized results between different assays. 38c. Your laboratory receives and analyzes proficiency test samples. In this case, 50 different laboratories in the United States (including yours) are provided with the same proficiency test sample and asked to measure the level of the CA-19.9 tumor marker. Half (i.e., 25) of the laboratories use assay No. 1 and obtain a mean of 36.5 (with a standard deviation [SD] of 4), whereas the other 25 laboratories use assay No. 2 and obtain a mean of 175.3 (SD 14). Which one of the following is the best explanation for the differences in performance between assay No. 1 and assay No. 2? B. Analytical sensitivity at the low end. C. High precision. D. Minimal analytical interferences from drugs. E. Lack of standardization among different assays. 38d. You are asked to implement an assay to measure CA-15.3. For monitoring the effectiveness of chemotherapy (i.e., for comparing pre- and posttreatment results) in an individual breast cancer patient, which one of the following performance characteristics for the CA-15.3 assay would be most important? B. Analytical sensitivity at the low end. C. High precision. D. Minimal analytical interferences from drugs. E. Lack of standardization among different assays. 39. Which one of the following statements best describes type II cryoglobulinemia? A. Detection of a monoclonal protein in the cryoprecipitate. B. Detection of albumin and a monoclonal protein in the cryoprecipitate. C. Detection of alpha-1, alpha-2, and beta globulins in the cryoprecipitate. D. Detection of polyclonal immunoglobulins in the cryoprecipitate. E. Detection of a monoclonal protein and polyclonal immunoglobulins in the cryoprecipitate. 40. Which one of the following laboratory results would be characteristic in a patient with Wilson disease? A. Low serum ceruloplasmin and low urinary copper concentrations. B. Low serum ceruloplasmin and elevated urinary copper concentrations. C. Elevated serum ceruloplasmin and low urinary copper concentrations. D. Elevated serum ceruloplasmin and elevated urinary copper concentrations. E. Normal serum ceruloplasmin and low urinary copper concentrations. 41. Which one of the following tumor markers is best for monitoring patients with breast cancer when used in conjunction with imaging studies? B. BRCA1/2. C. BRCA1 only. D. CA-15.3. E. EGFR genotyping. 42. Which one of the following tumor markers is best for monitoring patients with ovarian cancer when used in conjunction with imaging studies? B. HE4. C. BRCA1/2. D. CA-15.3. E. EGFR genotyping. 43. Which one of the following two tumor markers (and/or diagnostic modalities) is best for monitoring patients with prostate cancer? B. PSA density combined with PC3. C. PSA combined with PC3. D. PSA combined with circulating tumor cells (CTCs). E. No two modalities together; PSA alone is best. 44. Which one of the following statements is correct regarding Figure 8-3? A. Urine protein electrophoresis is not required to assess patients with hypogammaglobulinemia. B. Urine protein electrophoresis should not be performed using an aliquot from a random urine collection. C. Bence-Jones proteinuria is a diagnostic marker for multiple myeloma. D. The monoclonal light chains are found in concentrated urine in very small amounts and cannot be quantified as an M-spike by protein electrophoresis. E. Determination of serum free light chains is sufficient to diagnose Bence-Jones proteinuria. 45. Which one of the following answers best describes the findings seen in the three lanes in this serum protein electrophoresis gel shown in Figure 8-4? A. Hypergammaglobulinemia in lane 1; increased albumin in lanes 2 and 3. B. No abnormalities in lane 1; IgG kappa in lane 2; IgA lambda in lane 3. C. Bisalbuminemia in lane 1; abnormal band in the gamma region in lanes 2 and 3. D. Hypogammaglobulinemia in lane 1; hypergammaglobulinemia in lanes 2 and 3. E. No abnormalities in lane 1; IgA lambda in lane 2; IgG kappa in lane 3. 46. Which one of the following represents the most likely clinical scenario for a patient presenting with the “cryocrit” shown in Figure 8-5? A. A 22-year-old woman after a normal vaginal delivery. B. A 45-year-old patient with diabetes mellitus. C. A 45-year-old patient with hepatitis A. D. A 45-year-old patient with hepatitis C. E. A 5-year-old child with paroxysmal cold hemoglobinuria. 47. Which one of the following is the correct statement about the transport and processing of a sample for the determination of the “cryocrit” shown in Figure 8-6? A. Transport at room temperature and incubate at room temperature. B. Transport at 37°C and incubate at 4°C. C. Transport at room temperature and incubate at 4°C. D. Transport at 4°C and incubate at 37°C. E. Transport at 4°C and incubate at 4°C. 48. Monoclonal IgG proteins are most likely found in which one of the following fractions shown in Figure 8-7? B. Beta fraction. C. Gamma fraction. D. Alpha-1 fraction. E. Alpha-2 fraction. 49. Which one of the following is the best diagnosis determined by analyzing the serum protein electrophoresis results shown in Figure 8-8? A. Lane 7 demonstrates the presence of hypogammaglobulinemia. B. Lane 14 demonstrates the presence of increased amounts of polyclonal immunoglobulins. C. Lane 12 demonstrates a band in the gamma region that represents fibrinogen. D. Lane 18 demonstrates the presence of polyclonal hypergammaglobulinemia. E. Lane 20 demonstrates hypoalbuminemia. 50. Which one of the following statements about monoclonal immunoglobulins is true about Figure 8-9? B. The circulating concentration can be so high that the precipitating complexes by IFE will not stain properly. C. Quantitative monitoring clearly differentiates a benign from a malignant condition in the majority of cases. D. They are routinely detected using mass spectrometry methods. E. Identification of the constituent heavy chain and light chain types can be determined by IFE. 51. The fractions seen on a serum protein electrophoresis gel are separated from the anode (positive pole) to the cathode (negative pole). Which one of the following best represents this order of separation in Figure 8-10? A. 1. Albumin; 2. gamma fraction; 3. alpha-1; 4. alpha-2; 5. beta-1; 6. beta-2. B. 1. Gamma fraction; 2. alpha-1; 3. alpha-2; 4. beta-1; 5. beta-2; 6. albumin. C. 1. Albumin; 2. alpha-1; 3. alpha-2; 4. beta-1; 5. beta-2; 6. gamma fraction. D. 1. Alpha-1; 2. alpha-2; 3. beta-1; 4. beta-2; 5. gamma fraction; 6. albumin. E. 1. Gamma fraction; 2. alpha-1; 3. alpha-2; 4. beta-1; 5. albumin; 6. beta-2. 52. Which one of the following best describes rheumatoid factor (RF)? A. RF is an antigen that is used as a biomarker for the detection of rheumatoid arthritis. B. RF consists of antigens that are bound to the Fc portion of IgG. C. RF can cause a false-positive result with some immunochemical assays that measure hormones and drugs. D. The diagnostic specificity of RF for the detection of rheumatoid arthritis is greater than anti-cyclic citrullinated peptide (anti-CCP). E. Elevated serum RF levels are seen in most patients with rheumatoid arthritis. 53. Which disease is most commonly associated with the anti-nuclear antibody (ANA) staining pattern shown in Figure 8-11? B. Systemic sclerosis/CREST syndrome (calcinosis, Raynaud phenomenon, esophageal dysmotility, sclerodactyly, and telangiectasia). C. Systemic lupus erythematosus (SLE). D. Drug-induced (procainamide and/or hydralazine) lupus-like syndrome. E. Chronic active hepatitis. 54. Which one of the following diseases can be associated with the ANA staining pattern shown in Figure 8-12? B. Systemic sclerosis/CREST syndrome (calcinosis, Raynaud phenomenon, esophageal dysmotility, sclerodactyly, and telangiectasia). C. SLE. D. Asthma. E. Chronic active hepatitis. 55. Which disease is most commonly associated with the ANA staining pattern shown in Figure 8-13? B. Systemic sclerosis/CREST syndrome (calcinosis, Raynaud phenomenon, esophageal dysmotility, sclerodactyly, and telangiectasia). C. SLE. D. Drug-induced (procainamide and/or hydralazine) lupus-like syndrome. E. Sjögren syndrome. 56. Which disease is most commonly associated with the ANA staining pattern shown in Figure 8-14? B. Systemic sclerosis/CREST syndrome (calcinosis, Raynaud phenomenon, esophageal dysmotility, sclerodactyly, and telangiectasia). C. SLE. D. Drug-induced (procainamide and/or hydralazine) lupus-like syndrome. E. Many antigens and connective tissue diseases. Major points of discussion ■ Biopsy of prostate tissue is the gold standard for the diagnosis of prostate cancer. ■ PSA is a protease that circulates in the blood bound to protease inhibitors, and free PSA levels refer to the portion of PSA that is not bound to such proteins. ■ Prostatic volume can be determined through imaging studies, and density measurements can be obtained using imaging and measurement of PSA levels. ■ The measurement of PSA, along with digital rectal exam (DRE), is approved by the FDA for prostate cancer screening. 2. A. Carcinoembryonic antigen (CEA). Major points of discussion ■ The CA-125 epitope is an external fragment of a glycoprotein expressed on ovarian epithelial cells. ■ CEA is a large glycoprotein, and its measurement in serum is useful in the management of patients with gastrointestinal and pancreatic cancers. It can also be elevated in smoking and other benign conditions. ■ CA-19-9 is most useful in the management of patients with adenocarcinoma of the pancreas. It has been found not to be useful in patients with colon cancer. ■ AFP is an oncodevelopmental antigen that was one of the first tumor markers used in clinical practice. It is expressed in the fetus and is elevated in the serum of patients with HCC. ■ hCG is the “pregnancy hormone,” but it is also elevated in germ cell and pancreatic cancers. 3. A. PSA with DRE for prostate cancer. Major points of discussion ■ Mammography is recommended for breast cancer screening. ■ Testing for BRCA1/BRCA2 mutations is recommended in individuals with a family history of breast and ovarian cancers. ■ The majority of tumor markers are useful in monitoring therapy because they serve as surrogate markers of tumor burden. ■ AFP-L3 is a glycosylated form of AFP and is useful in the monitoring of therapy for HCC. 4. A. Brain natriuretic peptide (BNP) and N-terminal-prohormone BNP (NT-ProBNP). Major points of discussion ■ Urodilatin is a 36–amino acid peptide likely produced in the kidney. It regulates water and sodium reabsorption in the kidneys. It is not detected in blood. ■ BNP and NT-ProBNP are formed when the 134–amino acid pre-proBNP hormone is cleaved in myocytes to form the 108–amino acid proBNP molecule, along with the 26–amino acid signal peptide. ■ ProBNP is cleaved by the enzyme corin to form two polypeptides: an inactive 76–amino acid, NT-ProBNP, and the bioactive 32–amino acid, BNP. NT-ProBNP is a linear molecule, whereas BNP is horseshoe shaped. The ring structure is essential for the biological activity of the natriuretic peptides. ■ Both BNP and ANP are biologically active. ANP is usually not measured in serum because of its short half-life. BNP has a circulating half-life of 20 minutes, and NT-ProBNP has a circulating half-life of 1 to 2 hours. Circulating levels of NT-ProBNP are higher than those of BNP, and the values are not interchangeable.19,25 5. A. Elevated BNP or NT-ProBNP levels can be used to detect an acute myocardial infarction. Major points of discussion ■ BNP and NT-ProBNP levels are used to rule out heart failure and are extremely helpful when the cause of dyspnea is not clear. ■ Individuals with higher BNP or NT-ProBNP levels on admission to the hospital have a worse prognosis than those with lower BNP or NT-ProBNP levels. ■ A significant reduction in BNP or NT-ProBNP levels during hospitalization predicts a better outcome. However, the change needed to improve prognosis must be between 50% and 80% because of the large biological variation in BNP and NT-ProBNP levels. ■ Congestive heart failure can be ruled out if the BNP or NT-ProBNP levels are normal. ■ BNP and NT-ProBNP are elevated in noncardiac disorders, such as acute or chronic renal failure, sepsis, liver cirrhosis with ascites, and Cushing syndrome.19,25 6. A. CK-MB is detected in the blood before myoglobin after acute myocardial infarction. Major points of discussion ■ CK is essential for cellular metabolism and is found in all tissues. The highest CK activity is found in skeletal muscle because of its physiological role in maintaining the ATP levels required for muscle contraction. ■ CK-MM is the predominant isoenzyme in skeletal muscle, and CK-MB is the predominant isoenzyme in cardiac muscle. However, small amounts of CK-MB are detected in the red fibers, such as those in the soleus and intercostal muscles. Although the amount of CK-MB in skeletal muscle is small, CK-MB will be released when there is skeletal muscle damage; this may result in elevated CK-MB levels that incorrectly suggest cardiac damage. ■ The kinetics of circulating CK-MB levels after an acute myocardial infarction are as follows: CK-MB levels in blood increase at 4 to 6 hours, peak at 10 to 24 hours, and return to baseline levels at 48 to 72 hours. Serial determinations of CK-MB enhance its efficiency for the diagnosis of acute myocardial infarction. CK-MB levels are usually measured at 4- to 6-hour intervals. ■ Elevated circulating CK-MB levels can be observed following skeletal muscle trauma such as that seen in Duchenne muscular dystrophy, polymyositis, alcoholic myopathy, and marathon runners after a race. Therefore, CK-MB is not a completely specific biomarker for cardiac damage.10,18 7. A. Macromolecular complexes consisting of CK-MB bound to IgG. Major points of discussion ■ In an acute myocardial infarction, the ratio of CK-MB2 to CK-MB1 in serum exceeds 1.5 within 6 hours after the onset of symptoms. ■ The advantage of measuring CK- MB isoforms is that an acute myocardial infarction can be detected earlier by this approach, compared with measuring CK-MB alone. ■ The disadvantage of measuring CK-MB isoforms to diagnose an acute myocardial infarction is that they have the same specificity as CK-MB and can be elevated in skeletal muscle disease. ■ CK-MB isoforms are measured by a high-resolution electrophoresis procedure. This method is labor intensive and may not be able to detect small changes in CK-MB isoform concentrations. It also requires careful interpretation of the CK-MB isoform pattern. ■ An immunochemical procedure for measuring CK-MB isoforms was developed, but there was cross-reactivity with CK-MM.24 8. A. C3. Major points of discussion ■ CRP begins to increase at approximately 6 to 12 hours after the onset of a bacterial infection and usually peaks at 48 hours. CRP concentrations are usually higher in bacterial than in viral infections. CRP is used in the assessment of inflammatory disease, such as rheumatoid arthritis, neonatal sepsis and meningitis, malignancy, and trauma. ■ The magnitude of the increase in CRP levels is related to the severity of the inflammation and is the result of increased cytokine production, especially interleukin (IL)-6, which increases CRP synthesis. ■ CRP levels in serum are used to monitor a patient’s response to antibiotic treatment for bacterial infection. ■ Measurement of CRP levels is also used as an indicator of risk for cardiovascular disease.13 9. A. Measurement of troponin, myoglobin, and CK-MB. Major points of discussion 2. Changes on an electrocardiogram consistent with new ischemia, new ST-segment/T-wave changes, or new left bundle branch block. 3. Development of new pathologic Q waves on the electrocardiogram. 4. Imaging evidence of a new loss of viable myocardium or of a new regional wall motion abnormality. ■ If troponin is not available, CK-MB can be used as the biomarker.32 10. A. Troponin I. Major points of discussion ■ The JUPITER study tested the hypothesis that daily treatment with a statin (20 mg of rosuvastatin) compared with placebo would decrease the rate of the first major cardiovascular event in individuals who had signs of a low-grade inflammatory response indicated by an hsCRP level of greater than 2 mg/L and a low-density lipoprotein cholesterol (LDL-C) level of less than 130 mg/dL, which is below the current treatment threshold for a statin. ■ The JUPITER study was conducted in 17,802 apparently healthy persons from 1315 sites in 26 countries. The main baseline data showed an average LDL-C level of 108 mg/dL and an hsCRP level of 4.2 mg/L. ■ In the JUPITER study, rosuvastatin reduced LDL-C levels by 50% and hsCRP levels by 39%. The occurrence of an adverse cardiovascular event was reduced by 44% compared with placebo. The occurrence of myocardial infarction was reduced by 54% and that of unstable angina by 47%. ■ The JUPITER study demonstrated that asymptomatic individuals without hyperlipidemia, but with hsCRP levels greater than 2 mg/L, benefited from statin therapy. Because of this study, there has been a significant increase in CRP testing in clinical laboratories.26 11. A. Ischemia-modified albumin (IMA) and glycogen phosphorylase BB. Major points of discussion ■ Although both IMA and glycogen phosphorylase BB can be used to identify cardiac ischemia, IMA is the most frequently used biomarker for this purpose. ■ During cardiac ischemia, the N-terminus of albumin is altered, probably through a series of chemical reactions involving free radical damage to albumin. As a result, IMA is not able to bind metals such as cobalt. When albumin circulating in the blood comes in contact with ischemic tissue in the heart, some of it is converted into IMA. ■ IMA is produced continually during ischemia. IMA levels in blood rise quickly and remain elevated during an ischemic event. Ischemic patients have proportionally more IMA than do nonischemic patients. ■ IMA is not completely specific for cardiac ischemia. Elevated IMA levels can be obtained in any type of ischemia—for example, in brain ischemia and gastrointestinal ischemia. IMA is a marker for any ischemic event. ■ A normal IMA level has a high negative predictive value for patients who are being evaluated for acute coronary syndrome.4 12. A. Concurrent cardiac ischemia. Major points of discussion ■ In normal serum, LDH-2 is the most common isoenzyme, and the LDH-1/LDH-2 ratio is usually less than 1. The LDH-1 fraction will be greater than the LDH-2 fraction (i.e., “a flipped LDH-1/LDH-2 pattern”) if (1) hemolysis is present or (2) it has been several days since an acute myocardial infarction. ■ Haptoglobin is an acute-phase protein. Haptoglobin levels are decreased when there is hemolysis because the released hemoglobin binds haptoglobin and is rapidly cleared from the circulation. ■ During hemolysis, significant amounts of LDH-1 isoenzyme are released from the erythrocytes, resulting in a flipped LDH-1/LDH-2 pattern. ■ A flipped LDH-1/LDH-2 pattern is not a specific marker for myocardial necrosis.8,27 13. A. Elevated circulating myoglobin levels are diagnostic for an acute myocardial infarction. Major points of discussion ■ Myoglobin is present in both cardiac and skeletal muscle and, therefore, is not a specific cardiac marker. Because of its low molecular weight, myoglobin is rapidly released by cells into the circulation and is the first marker to be elevated after an acute myocardial infarction. ■ After an acute myocardial infarction, the serum levels of myoglobin increase between 1 and 4 hours, peak between 5 and 9 hours, and return to baseline levels between 24 and 36 hours. Serial determinations of myoglobin improve the predictive value of using a single myoglobin measurement to identify cardiac muscle injury. ■ The advantages of measuring myoglobin are that it is elevated in serum before other cardiac markers and that myoglobin serum concentration is dependent on the amount of cardiac damage. ■ The disadvantage of measuring myoglobin in the setting of a suspected acute myocardial infarction is that myoglobin is not a specific marker for cardiac necrosis; it is also increased in any condition where there is skeletal muscle damage (e.g., after cardiopulmonary resuscitation) and in renal failure. ■ A normal serum myoglobin value has a strong negative predictive value for an acute myocardial infarction in a patient admitted to the emergency department 6 hours after the onset of chest pain.12 14. A. Troponin T, calcium, and actin. Major points of discussion ■ Troponin C is an 18-kDa protein that binds calcium. ■ Troponin I is a 26.5-kDa actomyosin-ATP–inhibiting protein. ■ Troponin T is a 39-kDa protein that binds tropomyosin. ■ Troponin I and troponin T are present in the cytosolic pool at levels of 2% to 4% and 6% to 8%, respectively.17 15. A. Some troponin I methods are not accurate or precise. Major points of discussion ■ There at least seven different molecular forms of troponin I that are present in blood. Each immunochemical method uses different antibodies to measure each of the different molecular forms of troponin I. This results in significantly different troponin I results when comparing different methods. ■ Some of the molecular forms of troponin I that have been detected in blood are free troponin I, troponin I–troponin C complex, troponin I–troponin C–troponin T complex, reduced and oxidized forms of troponin I, and phosphorylated and nonphosphorylated forms of troponin I. ■ Each troponin I method has a different cutoff level for detecting an acute myocardial infarction. ■ The sensitivity and specificity of the troponin I methods for detecting an acute myocardial infarction are each approximately 98%.16 16. A. The troponin complex consists of cardiac troponin T, I, and C subunits that are structurally distinct from their skeletal muscle counterparts. Major points of discussion ■ The amino acid sequences of cardiac troponins I and T differ from those of skeletal muscle troponins I and T, respectively. Therefore, immunochemical methods can be developed that use antibodies to the cardiac-specific portions of the troponin I and troponin T molecules. ■ Elevated troponin I and troponin T levels are detected in serum 4 to 6 hours after an acute myocardial infarction and can remain elevated up to 4 to 7 days afterward. ■ There is a small amount of troponin I and troponin T in the cytosolic pool, which is released after an acute myocardial infarction as a result of cellular damage; this is responsible for the elevated troponin levels detected in blood 4 to 6 hours after an acute myocardial infarction. ■ Troponins I and T are slowly released from the myofibril after an acute myocardial infarction; this is responsible for the sustained, elevated troponin levels seen for several days after an acute myocardial infarction. ■ CK-MB is not completely cardiac specific and can also be detected in blood after skeletal muscle injury.3 17. A. Bilirubin interferes with the troponin T method. Major points of discussion ■ Troponin I is not expressed in fetal skeletal muscle. ■ Reference ranges for troponin T have not been established for newborns. ■ Reference ranges for troponin I have been established in newborns. ■ Cardiac troponin I has an additional 31 amino acids at the N-terminal end of the molecule compared with skeletal muscle troponin I. Troponin I has complete cardiac specificity and, therefore, this assay is used to determine if there is cardiac damage in newborns.5,6 18. A. The troponin T method can detect more patients with an acute myocardial infarction than the troponin I method. Major points of discussion ■ Although there are many molecular forms of troponin T in blood, only one immunochemical method, which uses one set of antibodies, is currently available to measure troponin T. Therefore, the troponin T results from different hospitals should be essentially the same because the same species of troponin T is being measured. ■ The general protocol used for evaluating patients admitted to the emergency department with chest pain is to measure troponin T on admission and at 3- to 6-hour intervals. ■ Elevated levels of troponin T are usually detected in serum by 4 to 6 hours after an acute myocardial infarction. ■ The sensitivity of the troponin T assay for detecting an acute myocardial infarction is approximately 98%. The specificity of the troponin T assay for detecting an acute myocardial infarction is approximately 95%.2 19. A. Heterophilic antibodies in the sample. Major points of discussion ■ Imprecise assays exhibit a high coefficient of variation (CV) and lack reproducibility. ■ Heterophilic antibodies are human xenoantibodies produced against a different species of animal (e.g., mice; human anti-mouse antibodies [HAMA]). They can cause interference in immunoassays that use antibodies from that particular animal species for capture and/or detection. ■ A multiplex ELISA is not a standard methodology for quantification of analytes in clinical laboratory assays, although they have been developed and are used in research settings. ■ A “sandwich immunoassay” refers to the use of both capture and detection antibodies, which sandwich the antigen of interest. 20. A. Presence of a band in the beta globulin fraction at a concentration of 0.2 g/dL. Major points of discussion ■ The monoclonal spike (i.e., the M-spike) is a sharp peak, typically present in the gamma globulin fraction of serum after separation by protein electrophoresis, and is indicative of a single species of immunoglobulin molecule. Its presence at this concentration is diagnostic for multiple myeloma. ■ CRP is a positive acute-phase reactant that can increase more than 100-fold above its normal levels in the setting of inflammation or infection. It migrates in the alpha globulin fraction of serum after separation via protein electrophoresis. ■ “Prealbumin,” another name for the serum protein transthyretin, is not otherwise related to albumin. Prealbumin migrates ahead of the albumin peak in conventional agarose gel electrophoresis; therefore, this is how it was named. ■ Microalbumin refers to low concentrations of albumin that are present in urine. It is elevated in patients with kidney injury and kidney disease. 21. A. Increased albumin; increased alpha-1, alpha-2, and beta globulins; and normal gamma globulins. Major points of discussion ■ Acute inflammation is characterized by increased production of acute-phase proteins. ■ In acute inflammation, alpha-1-antitrypsin is the major protein that is responsible for the increase in the alpha-1 region of the serum protein electrophoresis pattern. Alpha-1-acid glycoprotein is also an acute-phase protein, increased levels of which may be seen in the alpha-1 region in patients with sepsis. ■ In acute inflammation, haptoglobin is the major protein that is responsible for the increase in the alpha-2 region of the serum protein electrophoresis pattern. Ceruloplasmin is also an acute-phase protein, increased levels of which may be seen in the alpha-2 region in patients with severe liver disease. ■ CRP is increased in acute inflammation, but its concentration is usually too low to be detected by serum protein electrophoresis.7 ■ Albumin is decreased in acute inflammation because of increased production of IL-6, which decreases albumin synthesis in the liver. ■ In acute inflammation, the beta and gamma globulin fractions in the serum protein electrophoresis pattern are usually normal.14 22. A. The yolk sac. Major points of discussion ■ Neural tube defects (NTDs) result from failure of the neural tube to close spontaneously between the third and fourth week of in utero development. NTDs usually occur before a woman knows she is pregnant. ■ The concentration of AFP in the fetal blood is highest at about 10 to 13 weeks of gestation and is in the mg/mL range. The concentration of maternal serum AFP levels is normally in the ng/mL range. ■ In open spina bifida and anencephaly, AFP levels are elevated because fetal blood, which contains high concentrations of AFP, is excreted into the amniotic fluid, where it is in contact with the maternal circulation. The result is an increase in maternal serum AFP levels. ■ Only maternal serum and amniotic fluid AFP levels are used to screen for NTDs. 23. A. This is the serum protein electrophoresis pattern that is seen in severe liver disease. Major points of discussion ■ The elevated protein band in the alpha-2 region is most likely due to alpha-2-macroglobulin. ■ Alpha-2-macroglobulin is a large protein with a molecular weight of 725 kDa that is not excreted into the urine. Alpha-2-macroglobulin is a protease inhibitor. ■ A serum protein electrophoresis pattern with a low albumin, elevated alpha-2 globulin, a normal or decreased gamma globulin fraction, and a low total protein concentration is usually indicative of nephrosis. ■ In nephrosis, albumin and other low molecular weight proteins are excreted into the urine and the hepatic synthesis of alpha-2-macroglobulin is increased to compensate for the decreased oncotic pressure. As a result, high levels of alpha-2-macroglobulin are observed in nephrosis. ■ Although a monoclonal protein can be detected in the alpha-2 region, it is a rare occurrence.30 24a. A. Sensitivity. 24b. A. Sensitivity. 24c. A. Sensitivity. 24d. A. Sensitivity. 24e. A. Sensitivity. Major points of discussion ■ Precision is an assessment of the repeatability (i.e., reproducibility) of an assay. It is measured by calculation of the CV. It is important to measure the interassay and intra-assay CV to determine precision. ■ Predictive values (positive predictive value and negative predictive value) are characteristics that are dependent on the prevalence of the disease in the population being tested. ■ Reference intervals are typically established using 120 samples from a nondiseased cohort and taking the range of values from the central 95% of this population—that is, excluding three samples from each extreme. ■ Negative predictive value is the likelihood that a negative test result excludes disease. ■ Sensitivity is the ability to detect true positives in a cohort of patients. ■ The CV is determined by taking replicate measurements of a control sample and then dividing the standard deviation of those results by their mean. It is expressed as a percentage. 25. A. Serum protein electrophoresis (SPEP) showed low albumin and slightly elevated gamma globulin levels and the possibility of a monoclonal protein in the gamma globulin region, which was identified by IFE as a monoclonal IgG lambda protein. Major points of discussion ■ Fibrinogen may be present in the “serum” sample if the blood has not been allowed to clot for a sufficient amount of time. If the patient is receiving an anticoagulant that prevents complete clotting, the sample is collected in a tube that contains an anticoagulant. ■ A narrow band in the SPEP can be confirmed to be fibrinogen by performing IFE using antibodies to fibrinogen or by treating the sample with thrombin followed by serum protein electrophoresis; in the latter case, the band will disappear. ■ At very high concentrations of CRP, a small band may appear in the gamma globulin region of the SPEP. ■ A narrow band may appear at the application point and in all lanes on the IFE gel because of the presence of pentameric IgM aggregates or from polymerized IgA. This sample can be treated with 2-mercaptoethanol to break up these polymers. An IFE on the treated sample will then determine whether a monoclonal protein is present. ■ In cases of severe hemolysis, formation of a hemoglobin-haptoglobin complex may appear as a monoclonal band in the beta globulin region of the SPEP. Visual inspection of the specimen will reveal gross hemolysis.29 26a. A. Acquisition of this mutation marks the transition from low-grade to high-grade cancer. 26b. A. Acquisition of this mutation marks the transition from low-grade to high-grade cancer. 26c. A. CA-15.3. 26d. A. CEA has poor sensitivity for the diagnosis of lung cancer; only 20% of lung cancers are diagnosed using this marker. Major points of discussion ■ Several tumor markers have been used for lung cancer, but none are ideal. The markers used include CEA, neuron-specific enolase (NSE), cytokeratin-19 fragments (CYFRA 21-1), progastrin-releasing peptide (ProGRP), squamous cell carcinoma antigen (SCCA), and chromogranin A. ■ The tumor markers listed above are primarily useful for differential diagnosis, prognosis, postoperative surveillance, monitoring therapy, and recurrence detection. ■ In recent years, the EGFR has been well characterized at the molecular level. Several genetic aberrations have been identified in patients with NSCLC, including the L858R and T790M mutations. ■ The most widely used tumor marker used for managing patients with lung cancer is CEA. However, it suffers from a lack of specificity because it can be elevated in many non–lung cancer conditions. 27. A. Anencephaly and myelomeningocele. Major points of discussion ■ Patients with anencephaly have very elevated AFP levels. False-negative results are rare because there is usually no overlap in AFP values between anencephaly and patients with AFP values in the reference range. ■ False-negative results for NTDs can be obtained in patients with open spina bifida. ■ Patients with closed spina bifida usually have AFP values that are in the reference range, because AFP does not leak from the fetal blood into the maternal circulation in this setting. ■ False-positive screening results for NTDs can be obtained in multiple disorders, including intrauterine fetal demise, intrauterine growth retardation, gastroschisis, oligohydramnios, omphalocele, and congenital nephrosis. ■ Approximately 95% of patients with an NTD have no family history of NTDs. ■ The incidence of open NTDs in the United States is approximately 1:1000 live births.11 28. A. Albumin and alpha-1-antitrypsin. Major points of discussion ■ Transferrin, the major iron-binding protein in serum, migrates in the beta-1 region of the serum protein electrophoresis pattern. It is a negative acute-phase protein. In acute inflammation, transferrin synthesis by the liver is decreased, resulting in low transferrin levels. ■ Albumin is the most abundant protein in normal serum. It is a negative acute-phase protein. In chronic infections and acute inflammation, decreased albumin levels are usually seen because of increased production of IL-6, which decreases albumin synthesis by the liver. ■ Haptoglobin migrates in the alpha-2 region of the SPEP pattern. It is a positive acute-phase protein that is increased in pregnancy and cirrhosis. ■ C3, a component of the complement system, migrates in the beta-2 region of the SPEP pattern. It is a positive acute-phase protein that is increased during inflammation. ■ Transthyretin and retinol-binding protein are also negative acute-phase proteins, but their concentration in serum is too low to be detected by routine SPEP. 29. A. Nuchal translucency (NT), free beta-hCG, and AFP. Major points of discussion ■ Patients with a high risk for fetal Down syndrome have a low PAPP-A level, a high free beta-hCG level, and a high NT measurement. ■ First-trimester maternal screening for fetal Down syndrome is usually followed by measuring maternal serum AFP levels in the second trimester to test for the presence of fetal NTDs. ■ First-trimester maternal screening has improved the detection rate for fetal Down syndrome. With a false-positive rate of 5%, the detection rate for fetal Down syndrome is 86%. ■ Integrated testing uses the maternal screening results of the first and second trimesters to calculate the risk of fetal Down syndrome. First-trimester maternal screening followed by the maternal quadratic screen in the second trimester increases the detection rate for fetal Down syndrome to 95%, with a false-positive rate of 5%. ■ PAPP-A is a zinc-containing metalloproteinase glycoprotein that is produced by the trophoblast. PAPP-A interacts with insulin-like growth factor binding proteins to release insulin-like growth factors, which promote fetal growth and development.20 30. A. Vitamin A. Major points of discussion ■ Folic acid deficiency is associated with an increased risk of NTDs. ■ In 1998, the U.S. Public Health Service required that folic acid be added to breakfast cereals, infant formulas, pasta, rice, flour, and cornmeal. ■ After the fortification of foods with folic acid, the prevalence of NTDs in the United States decreased by 36%, from 10.8 per 10,000 population during 1995–1996 to 6.9 at the end of 2006. ■ Fortification of foods with folic acid is effective in preventing NTDs, because folic acid is accessible to all women of childbearing age without requiring any behavioral changes. ■ The Centers for Disease Control and Prevention recommends that all women of childbearing age take a multivitamin with 0.4 mg of folic acid per day. Women with a previous history of NTDs should take 4.0 mg of folic acid per day.9 31. A. Increased, because the MOM will be higher than expected. Major points of discussion ■ An incorrect gestational age is a common cause for an erroneous calculation of fetal NTD risk. ■ Calculation of the gestational age based on the last menstrual period is not always accurate and is the major reason physicians call the laboratory to ask for a recalculation of NTD risk based on a different gestational age. ■ Calculation of gestational age based on ultrasound is the preferred method. ■ Overestimation of gestational age will result in a lower calculated risk for a fetal NTD. ■ Underestimation of gestational age will result in a higher calculated risk for a fetal NTD. ■ MSAFP levels can increase by approximately sevenfold in anencephaly and approximately fourfold in open spina bifida. 32. A. Increased, because the actual MOM is higher than the calculated MOM. Major points of discussion ■ A positive AFAFP result is confirmed by measuring fetal hemoglobin levels and acetylcholinesterase in the amniotic fluid. ■ Detection of fetal hemoglobin in amniotic fluid as a result of amniocentesis can correlate with elevated AFAFP levels, because AFP is present in fetal blood at high concentrations. ■ Cerebrospinal fluid contains the enzyme acetylcholinesterase, which is specific to neural tissue but normally absent from amniotic fluid. In a case of open spina bifida, acetylcholinesterase leaks into the amniotic fluid. Detection of acetylcholinesterase in amniotic fluid is most likely the result of a fetal NTD. ■ In normal pregnancies, AFAFP levels decrease with increasing gestational age. Inaccurate estimation of gestational age will hinder the provision of an accurate risk of a fetal NTD.22 33. A. Increased serum MSAFP level and increased risk of NTDs. Major points of discussion ■ Because the serum MSAFP level is lower than expected in overweight patients, this results in a lower risk for NTDs if the weight correction is not performed. ■ The serum MSAFP levels increase with decreasing maternal weight because the maternal plasma volume is lower in underweight patients. ■ Because the MSAFP level is higher than expected in underweight patients, this results in a higher risk for NTDs if the weight correction is not performed. ■ In general, overweight women have an increased risk for open spina bifida.34 34. A. Proteins have a positive charge and migrate toward the anode. Major points of discussion ■ Proteins are amphoteric because of ionization of the acidic and basic side chains of amino acids. ■ At a pH of 8.6, serum proteins are negatively charged and, when placed in an electric field, they migrate towards the anode (i.e., positive electrode). ■ Albumin migrates the fastest and gamma globulins the slowest. The proteins in the alpha-1, alpha-2, and beta globulin regions migrate between albumin and the gamma globulins. ■ Electrophoresis is the migration of charged particles in an electric field. The rate of migration of proteins depends on the charge of the molecule, the size and shape of the molecule, the applied voltage, the nature of the support medium, and the pH and ionic strength of the buffer. ■ When the pH of a buffer solution equals the isoelectric point of the protein, the protein has no net charge and does not migrate in an applied electric field. When the pH of the solution is above the isoelectric point of the protein, the protein has a negative charge and migrates toward the anode.15 35. A. Elevated AFP, hCG, and inhibin A; low uE3. Major points of discussion ■ When a fetus has Down syndrome, AFP and uE3 concentrations are low and hCG and inhibin A levels are high. ■ An accurate gestational age is important in calculating the risk for Down syndrome, because overestimation of gestational age in a normal pregnancy can yield the same quadratic screening pattern that is seen when a fetus has Down syndrome. ■ Although the serum biomarkers levels are corrected for maternal weight, the risk for Down syndrome is not significantly affected by maternal weight. ■ The definitive test for detecting Down syndrome is chromosome analysis (e.g., cytogenetics). ■ The unadjusted risk for Down syndrome at term is approximately 1:385.11,20 36. A. All cases of fetal Down syndrome are detected. Major points of discussion ■ Using the quadratic screen (i.e., AFP, uE3, hCG, and inhibin A), the detection rate for fetal Down syndrome is 81% with a false-positive rate of 5%. ■ Calculation of the correct gestational age is important for obtaining accurate risk assessments of Down syndrome. Ultrasound evaluation of the gestational age is now the standard approach. ■ The age of the mother is important in calculating the risk of fetal Down syndrome; this risk increases with increasing maternal age. ■ In women undergoing assisted reproduction, the age of the egg donor is used to calculate the risk of fetal Down syndrome.11,20 37. A. AFP is elevated; uE3 and hCG are normal. Major points of discussion ■ The unadjusted incidence of trisomy 18 is approximately 1:8000 at term. ■ Screening methods based on the quadratic screen can identify approximately 60% of affected pregnancies with a false-positive rate of less than 0.4%. ■ More than 90% of infants born with trisomy 18 die within the first year of life. ■ A significant number of pregnancies affected by trisomy 18 are lost between prenatal diagnosis and term. Approximately 72% of pregnancies diagnosed with trisomy 18 end in miscarriage or stillbirth.23 38a. A. Wide dynamic range. 38b. A. Wide dynamic range. 38c. A. Wide dynamic range. 38d. A. Wide dynamic range. Major points of discussion ■ An assay with high analytical sensitivity (i.e., low limit of detection) is important in situations where there is a need to measure low levels of the analyte of interest. ■ Standardization among different assays for the same analyte is important when comparisons are made using results from the different assays. This does not necessarily apply to situations for comparison of results using just one assay. ■ When monitoring a cancer patient with tumor marker(s), it is ideal to make such an assessment using pre- and posttreatment measurements with the same methodology/analyzer in the same laboratory. ■ Some tumor markers can also be used for noncancer purposes; examples include hCG for detecting pregnancy and AFP for assessing fetal development in quadratic screening. The requirements in performance characteristics for these situations may differ based on the intended use of the analyte measurement. 39. A. Detection of a monoclonal protein in the cryoprecipitate. Major points of discussion ■ Proper sample collection is important for detection of cryoglobulins. Blood samples should be collected in warm tubes, transported to the laboratory at 37°C, and immediately centrifuged. False-negative cryoglobulin results can be obtained if the blood sample is allowed to clot at temperatures less than 37°C. ■ Analysis for cryoglobulins involves storing the serum for 7 days at 4°C and examining the sample for the presence of a cryoprecipitate. If present, the cryoprecipitate is washed several times and redissolved, and serum protein electrophoresis and IFE are performed. ■ Type I cryoglobulinemia is defined as the presence of a monoclonal protein (usually IgG or IgM) in the cryoprecipitate, and is associated with multiple myeloma and Waldenström macroglobulinemia. Type I cryoglobulins occur in about 10% of all cryoprecipitates. ■ Type II cryoglobulinemia is defined as the presence of a monoclonal protein (usually IgG or IgM) together with polyclonal immunoglobulins in the cryoprecipitate, and is associated with hepatitis C virus (HCV) infection, autoimmune diseases, and non-Hodgkin lymphoma. ■ Type III cryoglobulinemia is defined as the presence of only polyclonal immunoglobulins (usually IgM and IgG) in the cryoprecipitate and is associated with rheumatoid arthritis, autoimmune diseases, and chronic infections.28,33 40. A. Low serum ceruloplasmin and low urinary copper concentrations. Major points of discussion ■ ATP7B is expressed in hepatocytes and is needed for biliary copper excretion and for copper incorporation into ceruloplasmin. Absence or malfunction of ATP7B results in copper overload and incorporation of copper into the brain, liver, and cornea. ■ Ceruloplasmin is a copper-containing enzyme that transports about 95% of the copper from the gastrointestinal tract to peripheral tissues for synthesis of copper containing enzymes. ■ In Wilson disease, serum ceruloplasmin levels are low; urinary copper levels are elevated; and Kayser-Fleischer rings may be seen in the cornea. An elevated hepatic copper level is the gold standard for diagnosis. In some patients with Wilson disease, normal serum ceruloplasmin levels can be obtained because ceruloplasmin is an acute-phase protein and is increased in the setting of inflammation. ■ Wilson disease may present as unexplained hepatitis, as cirrhosis, with acute hemolytic anemia, or as a neuropsychiatric disorder.1 41. A. CA-125. Major points of discussion ■ CA-15.3 levels also correlate with tumor burden. Thus, they are generally higher with advanced-stage disease and as the tumor proliferates. ■ The family of CA markers correspond to cancer antigens. Most are glycosylated proteins that were the target of monoclonal antibodies produced when mice were challenged after injection of tumor cells. ■ The CA-15.3 and CA-27.29 assays measure different epitopes on the same protein. ■ The CA-15.3 assay is available through different diagnostic companies. The assays report slightly different values. When following any one particular patient, it is important that the same assay be used for monitoring purposes. Results from different CA-15.3 assays should not be compared directly. 42. A. CA-125. Major points of discussion ■ CA-125 is approved by the FDA for monitoring ovarian cancer. ■ Approximately 80% of ovarian cancers are of the serous epithelial type. The remaining 20% are a mixture of the mucinous, clear cell, endometrioid, and undifferentiated subtypes. ■ HE4 is a newly characterized biomarker that is useful in monitoring for recurrence and disease progression of serous epithelial ovarian cancer. When using this marker, a change is considered significant if this value increases or decreases by 25% or greater. ■ Mesothelin (i.e., soluble mesothelin-related peptides) is also used as a tumor marker for serous types of epithelial ovarian cancer. ■ ROMA (risk of ovarian malignancy algorithm) is a recently approved test to determine whether a biopsy sample is likely to show a malignancy. It is an index combining circulating HE4 and CA-125 levels, in conjunction with menopause status. 43. A. PSA combined with DRE. Major points of discussion ■ PSA density is calculated after performing a transrectal ultrasound, calculating the prostate volume (length × width × height × 0.52), then dividing the serum PSA (in ng/mL) by this value. A value of 0.15 or greater is used to determine whether further workup (including biopsy) is required. ■ PSA velocity refers to the rate of change of PSA. It evaluates the increase in PSA values over a defined period of time. A high PSA velocity may signify a more aggressive, rapidly growing tumor. ■ The CTC assay involves counting the number of CTCs in whole blood, after a capture, detection, and imaging procedure. ■ The CTC assay is FDA approved for monitoring patients with metastatic prostate cancer, as well as for monitoring patients with metastatic breast or colorectal cancer. 44. A. Urine protein electrophoresis is not required to assess patients with hypogammaglobulinemia. Major points of discussion ■ Urine protein electrophoresis interpretation is challenging because it is often necessary to detect monoclonal immunoglobulins in the presence of significant proteinuria. Proteinuria is defined as more than 150 mg of urine protein in 24 hours; this is equivalent to a more than 0.15 protein/creatinine ratio in a random “spot” urine sample. ■ To determine the amount of monoclonal protein produced, it is necessary to analyze an aliquot obtained from a 24-hour urine collection. ■ Urine must be concentrated to identify its relevant protein fractions. The concentration of clinically relevant monoclonal light chains can be low in unfractionated urine. Therefore, urine must be concentrated up to 100-fold to visualize all protein fractions. The optimal concentration of protein in urine for appropriate visualization by electrophoresis is 25 to 50 mg protein/mL. Urine can be concentrated using solvent absorption devices, lyophilization, or protein spin columns. ■ Monoclonal light chains can occasionally be seen in serum, even though their serum half-life is only 2 to 6 hours. ■ Possible scenarios are as follows: • Production of large quantities of monoclonal protein by a light chain myeloma. • Monoclonal light chains can occasionally exist as homotetramers, which are then too large to be filtered through the glomerular basement membrane. • A reduced number of nephrons due to underlying renal disease, which reduces the clearance of monoclonal light chains. • Monoclonal light chains may bind to other serum proteins, which are then too large to be filtered through the glomerular basement membrane, and produce multiple bands by serum protein electrophoresis and IFE. 45. A. Hypergammaglobulinemia in lane 1; increased albumin in lanes 2 and 3. Major points of discussion ■ Homogeneous proteins are seen in the gamma region of both lanes 2 and 3. This finding strongly suggests the presence of a monoclonal protein. Immunofixation electrophoresis is required for identification of the abnormal proteins. It is not possible to know the type of immunoglobulin that constitutes the abnormal clone using only serum protein electrophoresis. ■ A monoclonal band of immunoglobulins indicates that a clonal population of B cells or plasma cells exists, which has escaped normal regulation and might result in a plasma cell dyscrasia, such as multiple myeloma. In contrast, a polyclonal distribution of immunoglobulins generally indicates a reactive process of an otherwise normal immune system. ■ Five different classes of antibody are known: immunoglobulin (Ig)M, IgG, IgA, IgD, and IgE, each with a distinct heavy chain. Two different light chain types have been identified: kappa and lambda. IgG is prevalent in blood and tissue fluids; IgM is found mainly in the blood; secretory IgA is found primarily on epithelial surfaces; IgD is mostly bound to the surface of B cells; and IgE is largely bound to the surface of basophils and mast cells. ■ Monoclonal increases in immunoglobulins suggest the presence of a plasma cell dyscrasia. Polyclonal increases in immunoglobulins occur as part of the immune response and may be found in chronic disease. 46. A. A 22-year-old woman after a normal vaginal delivery. Major points of discussion ■ To determine the type of cryoglobulinemia (i.e., types I, II, III), the cryoprecipitate is washed to remove any contaminating liquid serum remaining. The goal is to wash off all proteins that are not part of the precipitate. Washing up to 5 times with NaCl 0.9% is necessary to achieve a satisfactory result. The resulting pellet is then resolubilized and processed for IFE. ■ Type I cryoglobulins typically form a relatively large volume of cryoprecipitate (e.g., a cryocrit of up to 10%, or even 20%). Upon IFE, type I cryoglobulins exhibit a single heavy chain and a single light chain type. Type I cryoglobulinemia is associated with monoclonal gammopathy of undetermined significance, macroglobulinemia, or multiple myeloma. ■ Type II cryoglobulins typically show only a small amount of cryoprecipitate (i.e., less than 1% of the serum volume). Quantifying the cryocrit is not analytically valid for serial measurements to monitor this type of patient’s progress. IFE most often shows a monoclonal IgM band plus polyclonal IgG. Type II cryoglobulinemia is associated with autoimmune disorders, such as vasculitis, glomerulonephritis, systemic lupus erythematosus, rheumatoid arthritis, and Sjögren syndrome. It may be seen in infections such as hepatitis, infectious mononucleosis, cytomegalovirus, and toxoplasmosis. Type II cryoglobulinemia may also be “essential”; this is, it may occur in the absence of an underlying disease. ■ In type III cryoglobulinemia, IFE demonstrates both polyclonal IgM and polyclonal IgG. Type III cryoglobulinemia usually presents with only trace levels of cryoprecipitate, which may take up to 7 days to appear, and is associated with the same disease spectrum as type II cryoglobulinemia. ■ Potential causes for cryoglobulinemia: • Hepatitis C • Autoimmune disease • Other • Hematological malignancies • Unknown cause in approximately 10% of cases 47. A. Transport at room temperature and incubate at room temperature. Major points of discussion ■ Common causes of cryoglobulinemia are given in the Major Points of Discussion for Question 46. ■ Warm (37° to 40°C) serum samples are required for proper determination of cryoglobulinemia. If a patient’s blood sample is not maintained at this temperature before reaching the performing laboratory, the cryoglobulins may precipitate and settle on top of the red blood cell layer. Therefore, when the tube is centrifuged to separate the serum, the cryoglobulins form a sediment with the coagulated/cellular fraction. Rewarming the initial whole blood tube does not correct this preanalytical error because cryoglobulins that were trapped in the coagulated layer cannot be mobilized. ■ To achieve appropriate results, the blood sample obtained from the patient can be kept warm by wrapping the tube in a “heel warmer,” or the tube can be transported in a warm water bath. Failure to follow the relevant specimen-handling instructions may cause false-negative results. ■ The presence of albumin in a cryoprecipitate IFE gel indicates insufficient washing of the precipitate. Albumin is the major protein constituent in serum. If serum contamination is present, it is not clear whether visualized polyclonal immunoglobulins that define type II (mixed-type) and type III (polyclonal) cryoglobulinemia originate from the contaminating serum component or from the cryoprecipitate itself. ■ Testing for cryoglobulinemia is not useful for general population screening without a clinical suspicion of cryoglobulinemia. 48. A. Albumin fraction. Major points of discussion ■ Apo A1 (mainly found on high density lipoproteins), alpha-1 fetoprotein, and alpha-1 antitrypsin migrate in the alpha-1 fraction of a standard SPEP gel. ■ Alpha-2 macroglobulin, haptoglobin, and ceruloplasmin migrate in the alpha-2 region of a standard SPEP gel. ■ Fibrinogen and complement factors C3 and C4 migrate in the beta-2 region of a standard SPEP gel. The presence of fibrinogen in a sample can result in the false-positive detection of a band in the beta-2 region. ■ Haptoglobulin migrates in the alpha-2 region of a standard SPEP gel. In sera from patients with hemolytic anemia or other forms of hemolysis, some haptoglobin-hemoglobin complexes may be seen. 49. A. Lane 7 demonstrates the presence of hypogammaglobulinemia. Major points of discussion ■ Hypergammaglobulinemia can also be seen in infections, liver diseases (acute and chronic hepatitis, biliary cirrhosis, lupoid hepatitis), pulmonary disorders (sarcoidosis, berylliosis, pulmonary hypersensitivity syndrome), Down syndrome, amyloidosis, narcotic addiction, and renal tubular disease. ■ Monoclonal immunoglobulins or fragments of immunoglobulins have been associated with multiple disease conditions. These include: • Waldenström macroglobulinemia • Chronic lymphocytic leukemia • Other leukemias • Lymphomas • “Benign” monoclonal gammopathy • Systemic capillary leak syndrome • Amyloidosis • Chronic liver disease, such as chronic active hepatitis and primary biliary cirrhosis • Autoimmune disorders, including rheumatoid arthritis, systemic lupus erythematosus, thyroiditis, pernicious anemia, polyarteritis nodosa, Sjögren syndrome • Gaucher disease • Malignancies of various types • Hereditary spherocytosis • HIV infection, including AIDS ■ The term monoclonal gammopathy of undetermined significance (MGUS) categorizes individuals in whom a monoclonal component is demonstrated in the serum but who lack other key features for diagnosing a malignant condition. As many as 3% of individuals over the age of 70 have MGUS. Subjects with MGUS may have as many as 10% plasma cells in their bone marrow. The risk of progression to multiple myeloma in patients with MGUS is approximately 1% per year, even after 25 years of a stable condition. It is recommended that such patients be followed up with SPEP every 6 to 12 months to determine whether the process is progressing or regressing. ■ False-positive interpretations of monoclonal bands can occur owing to the presence of fibrinogen from an incompletely clotted specimen or when hemolysis occurs as the result of improper specimen collection. Confirmation that a faint band is a monoclonal paraprotein is typically done by using IFE. 50. A. At very low concentrations, they can significantly increase the viscosity of blood, leading to problems with organ perfusion. Major points of discussion ■ Requests to examine sera for the presence of a monoclonal protein usually are generated by a physician who recognizes that a patient has clinical symptoms and signs consistent with such a disorder. Alternatively, this is determined when laboratory examination of a SPEP gel suggests the presence of a monoclonal protein. Many times, the physician’s suspicion of a plasma cell dyscrasia is triggered by findings of anemia (with one or more cytopenias), elevated total serum protein with elevated globulins, and proteinuria; other findings may include hyperuricemia, hypercalcemia, elevated alkaline phosphatase, bone pain, or lytic lesions of bone on radiography. ■ If monoclonal proteins are detected by SPEP and IFE, it is then recommended to quantify the immunoglobulins. ■ Quantitative immunoglobulins are useful in monitoring the course of the disease and its treatment and may be helpful in separating a benign from a malignant condition. Monoclonal IgG levels of 2 g/dL, or IgA levels of 1 g/dL or greater, suggest a malignant condition. In many malignant immunocytopathies, the concentration of nonmonoclonal immunoglobulins is reduced. Thus, a deficiency of polyclonal immunoglobulins is also suggestive of malignancy. ■ Immunoglobulins at very high concentrations can significantly increase the viscosity of blood, leading to problems with organ perfusion (e.g., headache, visual disturbances, renal dysfunction). 51. A. 1. Albumin; 2. gamma fraction; 3. alpha-1; 4. alpha-2; 5. beta-1; 6. beta-2. Major points of discussion ■ Current approaches separate proteins at a pH of 8.6 on solid support media such as cellulose acetate membranes, agarose gels, starch gels, and polyacrylamide gels that can separate up to five fractions: albumin, alpha-1, alpha-2, beta, and gamma proteins. The beta fraction can also be subdivided into beta-1 and beta-2 fractions. ■ Proteins are large molecules composed of covalently linked amino acids. Depending on electron distributions resulting from covalent or ionic bonding of structural subgroups, proteins have different electrical charges at a given pH. Protein separation occurs due to size and charge. ■ A voltage is applied between the electrodes, generating a current that passes through the gel, usually for a period of approximately 30 minutes, to achieve the desired resolution. The ionic strength of the buffer determines the amount of current and the movement of the proteins for a fixed voltage. If ionic strength is low, relatively more current is carried by the charged proteins. If ionic strength is high, less current is carried by the proteins, which move a shorter distance. Through manipulations of buffer salt composition, endosmotic properties of the medium, and means of sample application, commercially available agarose plates now achieve consistently high-resolution quality that allows routine separation of all major serum protein species. ■ Albumin is the most abundant serum protein. It is found at the anodal (the positive pole) end of the gel. Immunoglobulins are found in the gamma fraction, which is most cathodal (the negative pole). ■ Proteins are negatively charged at a pH of 8.6 and migrate toward the anode. Exceptions include gamma globulins and some beta globulins, which migrate toward the cathode. The movement of cationic buffer ions toward the cathode pulls weaker charged proteins along; this process is called endosmosis. 52. A. RF is an antigen that is used as a biomarker for the detection of rheumatoid arthritis. Major points of discussion ■ RF is an autoantibody directed against the Fc region of IgG. Although IgM is the most common isotype of RFs, RF reactivity can also be detected in the IgA, IgG, and IgD subclasses. ■ The diagnostic specificity of anti-CCP is better than RF for the detection of rheumatoid arthritis. The combination of RF and anti-CCP has been proposed for detecting rheumatoid arthritis. ■ The most common methods for measuring RF are automated nephelometric or turbidimetric procedures. In these assays, polystyrene beads are coated with an immune complex consisting of human gamma globulin and sheep anti-human IgG. Agglutination occurs when a specimen containing RF is added to these beads. The amount of turbidity that is formed is determined by the intensity of the light scattered in nephelometric assays or by the amount of light absorbed in turbidimetric assays. ■ High levels of circulating RF can cause positive interferences with some free T4, thyroid-stimulating hormone (TSH), troponin I, and tacrolimus immunochemical assays. Thus, RF can bind to both the capture and labeled antibodies and produces a signal independent of the analyte that is being measured. This interference can be eliminated by adding animal immunoglobulins to the reagents, thereby preventing RF binding to the capture antibody. This is the same type of interference that can be caused by human anti-animal antibodies (i.e., HAAAs).21,31 53. A. Myositis. Major points of discussion ■ The standard method for ANA testing in the United States uses IFE analysis of HEp-2 cells. Mouse liver cells can also be used, but they are less sensitive than Hep-2 cells. Nondiseased individuals may exhibit ANA titers as high as 1:80 when HEp-2 cells are used. ■ ELISA-based methods can be used for screening purposes. However, the disadvantage of using such an approach is that this methodology cannot provide information on IF patterns, which have been the gold standard for decades. ■ There are three antibodies commonly associated with systemic scleroderma: (1) anti-topoisomerase I, (2) anti-RNA polymerase, and (3) anti-centromere. Thus, a speckled or nucleolar pattern may be observed. ■ Anti-centromeric antibodies are common (44% to 98%) in patients with CREST syndrome (a subset of scleroderma patients). CREST patients suffer from skin changes that are not systemic but are limited to the hands and face. 54. A. Sjögren syndrome. Major points of discussion ■ Results from anti-dsDNA antibody testing are elevated in a majority of patients with SLE (approximately 70% in one study and up to 90% at some point during the course of disease). dsDNA positivity usually produces a homogeneous or peripheral staining pattern in ANA testing. ■ The homogeneous (diffuse) pattern corresponds to specific nuclear antigens associated with chromatin, histones, and also DNA. ■ SLE patients usually exhibit a peripheral pattern, although homogeneous and sometimes speckled patterns have also been observed. ■ A homogeneous pattern is also observed in more than 90% of patients with drug-induced lupus-like syndrome (precipitated by procainamide or hydralazine). An important caveat to this testing is that multiple drugs can result in a false-positive ANA result. 55. A. Myositis. Major points of discussion ■ There are four basic staining patterns: (1) homogeneous (diffuse), (2) peripheral, (3) speckled, and (4) nucleolar. Each is characteristic of a different set of autoantigens that can produce ANAs. ■ It is important to note that the presence of any one pattern does not correlate 100% with any one connective tissue disease. However, reasonable generalizations can be made to classify groups of antigens based on the observed patterns. ■ The nucleolar pattern corresponds to proteins of the nucleolus including RNA polymerase. ■ Patients with Sjögren syndrome or scleroderma usually exhibit a nucleolar pattern with ANA testing. 56. A. Sjögren syndrome. Major points of discussion ■ The advantages of a reverse algorithm approach for ANA testing include that it (1) reduces labor (technologist time) required for screening all samples by IF and (2) provides for a quantitative and objective (vs. subjective) means to identify the positive samples that require further workup. ■ A speckled ANA pattern is shown in the figure. Although there are four basic staining patterns (homogeneous, peripheral, speckled, and nucleolar) revealed through IF analysis of ANA, it is important to note that the presence of any one pattern does not correspond 100% to any one disease group; only generalizations can be made to correlate distinct antigens with specific diseases. ■ The speckled pattern observed in ANA testing corresponds to non-DNA nuclear constituents, such as RNPs (ribonuclear proteins) or the Sm, SS-A, and SS-B antigens. ■ Of all the patterns, the speckled pattern is least specific. It has been observed with multiple connective tissue diseases.
Clinical Chemistry
Cardiac, Cancer, and Other Biomarkers
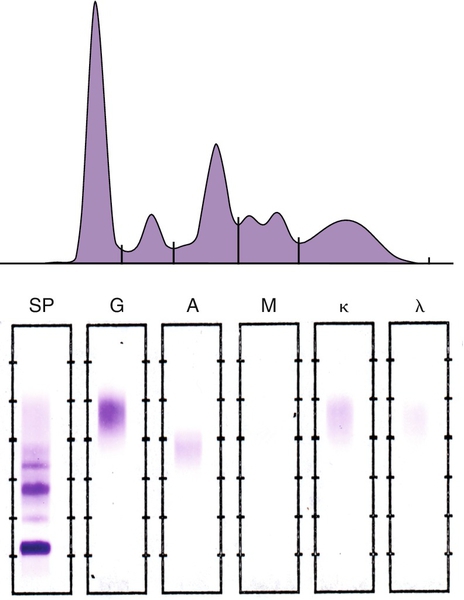
Protein Fractions
Patient Results (g/dL)
Reference Range (g/dL)
Albumin
2.2
3.6-4.8
Alpha-1
0.5
0.2-0.4
Alpha-2
1.4
0.6-0.9
Beta
0.9
0.6-1.0
Gamma
1.2
0.8-1.8
Total protein
6.2
6.7-8.6
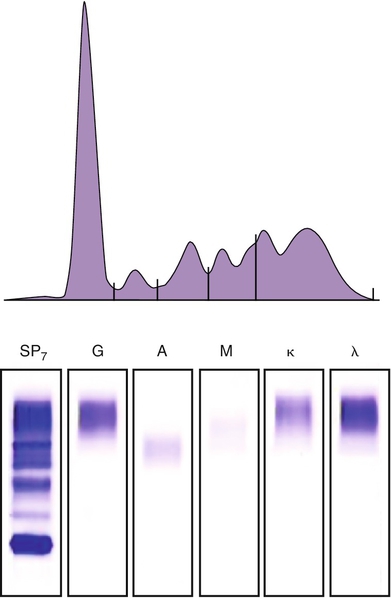
Protein Fractions
Results (g/dL)
Reference Range (g/dL)
Albumin
2.9
3.6-4.8
Alpha-1
0.3
0.2-0.4
Alpha-2
0.7
0.6-0.9
Beta
0.9
0.6-1.0
Gamma
2.2
0.8-1.8
Total protein
7.0
6.7-8.6
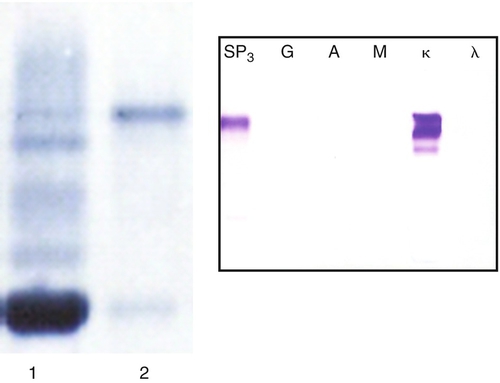
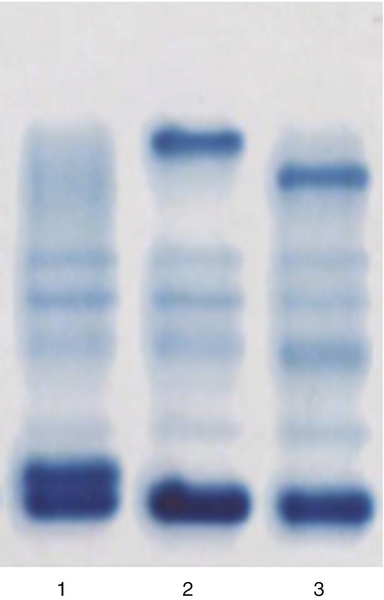
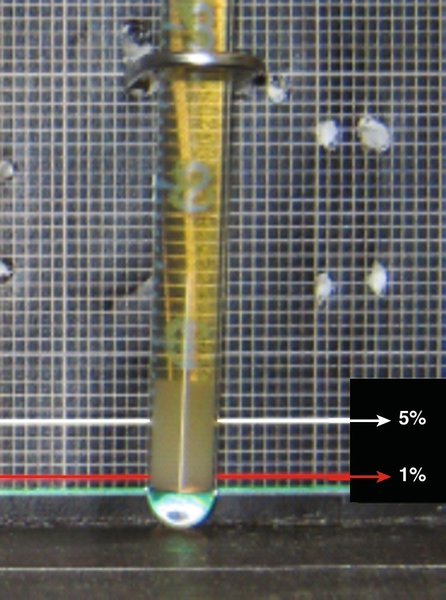

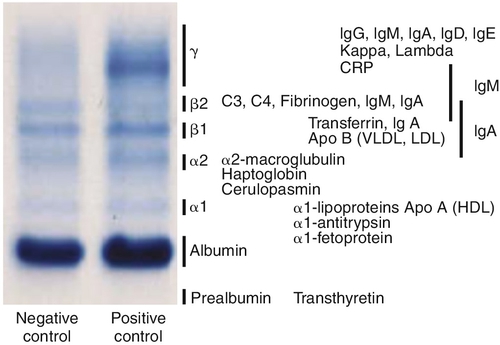
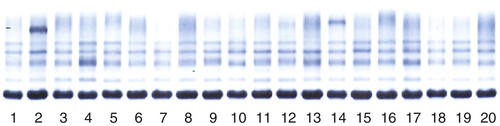
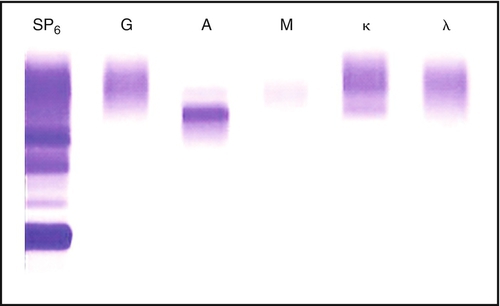
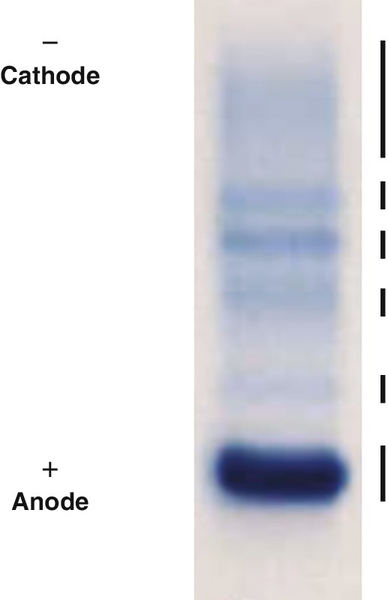
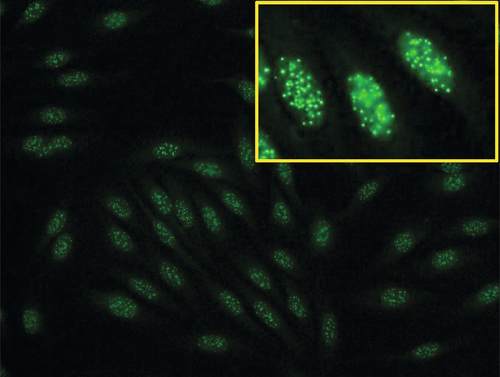
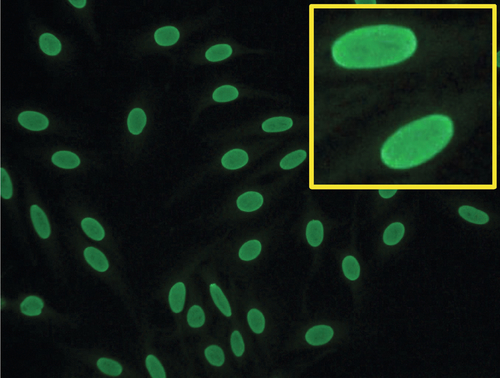
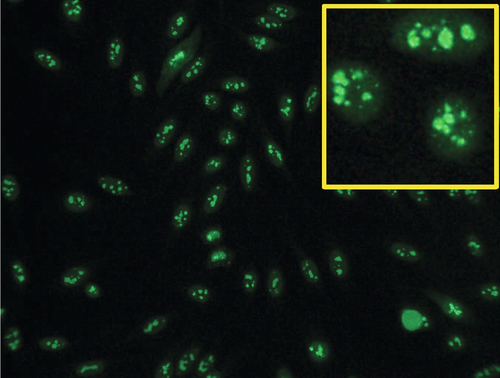
Rationale: CEA measurement in serum is useful in the management of patients with gastrointestinal and pancreatic cancers.
B. Carbohydrate antigen 19-9 (CA-19-9).
Rationale: CA 19-9 is useful in the management of patients with pancreatic cancer.
C. Cancer antigen 125 (CA-125).
Rationale: CA-125 is useful in the management of patients with ovarian cancer.
D. Alpha fetoprotein (AFP).
Rationale: AFP is useful in the management of patients with germ cell tumors or hepatocellular carcinoma (HCC).
E. Human chorionic gonadotropin (hCG) subunits.
Rationale: hCG subunits are useful in the management of patients with endocrine pancreatic tumors.
Rationale: PSA, with DRE, is FDA approved in the United States for screening of prostate cancer.
B. CA-125 and ultrasound imaging for breast cancer.
Rationale: CA-125 is useful in ovarian cancer, not breast cancer.
C. CEA for lung cancer in a patient with a history of smoking.
Rationale: CEA can be elevated above the reference range in smokers.
D. AFP, for HCC in a patient with elevated levels of aspartate and alanine aminotransferase.
Rationale: Although this combination may be elevated in HCC, other noncancerous conditions may also show elevation in these markers.
E. Serial hCG measurements for breast cancer.
Rationale: hCG has not been shown to be useful in the management of patients with breast cancer.
B. BNP and pre-prohormone of BNP.
C. Atrial natriuretic peptide (ANP) and BNP.
Rationale: Both are biologically active.
D. Urodilatin and BNP.
Rationale: Urodilatin is not biologically active.
E. ANP and N-terminal-prohormone BNP.
Rationale A, B, and E: NT-ProBNP and pre-proBNP are not biologically active.
B. Elevated BNP or NT-ProBNP levels can be used to detect unstable angina.
C. Elevated BNP or NT-ProBNP levels can be used to detect cardiac ischemia.
Rationale A, B, and C: BNP and NT-ProBNP are not used to detect an acute myocardial infarction, unstable angina, or cardiac ischemia.
D. Elevated BNP or NT-ProBNP levels can be used to screen for congestive heart failure in asymptomatic individuals.
Rationale: BNP and NT-ProBNP are not used as screening tests for asymptomatic congestive heart failure.
E. Normal BNP or NT-ProBNP levels can be used to rule out congestive heart failure.
Rationale: A normal BNP or NT-ProBNP level is a good negative predictor for congestive heart failure.
Rationale: After an acute myocardial infarction, myoglobin appears in the blood before CK-MB.
B. CK-MB is found only in cardiac muscle.
Rationale: CK-MB is found in both cardiac and skeletal muscle.
C. CK-MB is detected in blood before troponin I or troponin T after an acute myocardial infarction.
Rationale: After an acute myocardial infarction, CK-MB and troponin are detected in the blood at approximately the same time.
D. CK-MB is known to rise and fall predictably after an acute myocardial infarction.
Rationale: In the 1980s, CK-MB was the gold standard for detecting an acute myocardial infarction.
E. CK-MB is a better predictor than troponin I or T for risk stratification of patients with acute myocardial infarctions.
Rationale: Troponin is used for risk stratification in cardiac patients.
B. CK mitochondrial complexes.
Rationale: They are not mitochondrial isoenzymes.
C. Artifactual forms of CK caused by binding of CK to albumin.
Rationale: They are not bound to albumin.
D. Posttranslational modification of CK-MB.
Rationale: A lysine residue is removed from the CK-M subunit of CK-MB, which yields the two CK-MB isoforms.
E. CK complexes consisting of CK-MB bound to immunoglobulin A (IgA).
Rationale A and E: Immunoglobulins are not part of the CK isoforms.
Rationale: There is a small to moderate increase in C3 in bacterial infections.
B. C4.
Rationale: There is a small increase in C4 in bacterial infections.
C. Albumin.
Rationale: Albumin is a negative acute-phase protein.
D. C-reactive protein (CRP).
Rationale: There is a substantial increase in CRP after a bacterial infection.
E. Transferrin.
Rationale: Transferrin is a negative acute-phase protein.
B. Measurement of CK-MB and troponin.
Rationale: Measurement of both biomarkers is not recommended in this setting.
C. Measurement of myoglobin and troponin.
Rationale for A and C: Measurement of myoglobin is not recommended in this setting.
D. Measurement of the rise and fall of troponin.
Rationale: This is the recommended biomarker for acute myocardial infarction.
E. Measurement of CK-MB isoforms and troponin.
Rationale: Measurement of CK-MB isoforms is not recommended in this setting.
B. Ischemia modified albumin.
Rationale: This marker is used to rule out ischemia.
C. High-sensitivity CRP (hsCRP).
Rationale: This marker can be used to predict the risk of future cardiac events.
D. BNP.
Rationale: This is a marker of congestive heart failure.
E. CK-MB.
Rationale for A and E: These are the markers of myocardial necrosis.
Rationale: These are the markers that can be used to identify cardiac ischemia.
B. IMA and troponin.
Rationale: Troponin is used to identify myocardial necrosis, not cardiac ischemia.
C. IMA and free fatty acid binding protein.
Rationale: Fatty acid binding protein is a marker of myocardial necrosis.
D. IMA and BNP.
Rationale: BNP is a marker of congestive heart failure, not cardiac ischemia.
E. IMA and myosin light chain.
Rationale: Myosin light chain is a marker of myocardial necrosis, not cardiac ischemia.
B. Concurrent congestive heart failure.
C. Concurrent unstable angina.
Rationale: LDH-1 is usually not elevated in ischemia, congestive heart failure, or unstable angina.
D. Artifact due to hemolysis.
Rationale: A low to absent haptoglobin level and a high LDH-1 fraction usually indicate hemolysis.
E. Increasing liver dysfunction.
Rationale: LD4 and LD5 are the isoenzymes associated with liver function.
Rationale: Myoglobin is not a specific cardiac marker.
B. Circulating myoglobin levels are elevated for days after an acute myocardial infarction.
Rationale: Myoglobin is an early cardiac marker.
C. Circulating myoglobin levels become elevated after troponin I or troponin T after an acute myocardial infarction.
Rationale: Myoglobin is elevated before troponin.
D. Circulating myoglobin levels peak after CK-MB levels rise after an acute myocardial infarction.
Rationale: Myoglobin is elevated before CK-MB.
E. Normal circulating myoglobin levels can be used to rule out an acute myocardial infarction.
Rationale: In an acute myocardial infarction, myoglobin would be elevated 4 hours after the onset of chest pain.
B. Troponin C, myoglobin, and actin.
C. Troponin I, actin, and tropomyosin.
D. Troponin I, troponin T, and troponin C.
Rationale: The troponin complex consists of troponins I, C, and T.
E. Troponin T, tropomyosin, and calcium.
Rationale for A, B, C, and E: Calcium, actin, tropomyosin, and myoglobin are not part of the troponin complex.
Rationale: The currently available troponin I methods are very accurate and precise.
B. Some troponin I methods cross-react with troponin T.
C. Some troponin I methods are standardized.
Rationale: There is no standardization for troponin assays.
D. Some troponin I methods cross-react with troponin C.
Rationale for B and D: There is no cross-reactivity with troponin T or troponin C.
E. Some troponin I methods measure different molecular forms of troponin I.
Rationale: Many different immunochemical methods are used to measure troponin I, using different sets of antibodies, resulting in different analytical results.
Rationale: The cardiac troponin C and skeletal troponin C molecules have the same structure.
B. The troponin complex is not found in the cytosolic pool.
Rationale: There is some troponin in the cytosol.
C. Troponin is released early into blood after an acute myocardial infarction because of degradation of the contractile apparatus.
Rationale: Early release of troponin from the cytosolic pool is the reason why troponin is detected early after an acute myocardial infarction.
D. Troponin is part of the thick filament (myosin) of muscle.
Rationale: Troponin is bound to the myofibril structure.
E. Cardiac troponin T and I subunits are distinctly different from their skeletal muscle counterparts.
Rationale: Troponin I and troponin T are specific cardiac biomarkers, which are useful for detecting cardiac damage.
Rationale: There is no interference from bilirubin with the troponin T method.
B. Skeletal muscle troponin T found in newborns cross-reacts with troponin T.
Rationale: Fetal skeletal muscle contains some cardiac troponin T, which cross-reacts with the troponin T detection method.
C. Precision of the troponin T method in newborns has not been established.
Rationale: The precision of the troponin T method is known.
D. The troponin T method requires a large sample volume and is not suitable for use in newborn testing.
Rationale: The sample volume for measuring troponin T is small, less than 50 μL.
E. The analytical sensitivity of the troponin T method is not high enough and therefore cannot be used for newborn testing.
Rationale: A high-sensitivity method is available for measuring troponin T.
Rationale: There is no significant difference in the detection of an acute myocardial infarction using either the troponin I or troponin T methods.
B. Troponin T is more stable in blood than troponin I.
Rationale: There is no difference in stability between troponin I and troponin T in blood.
C. The troponin T method is more precise than the troponin I method.
Rationale: There is no significant difference in precision between the troponin I and troponin T methods.
D. Only one method is available to measure troponin T.
Rationale: There is only one immunochemical method from one vendor available to measure troponin T. In contrast, there are multiple methods for measuring troponin I.
E. Only one molecular form of troponin T is found in blood.Rationale: Many molecular forms of troponin T are found in blood.
Rationale: Although heterophilic antibodies can produce a falsely depressed value, this type of response is likely to be an all-or-none phenomenon. Thus, this is not the best answer to this question.
B. Imprecision of the assay.
Rationale: Imprecision would not necessarily result in a falsely low value; it would manifest as a lack of reproducibility of the assay when measuring samples.
C. Calibration using a sigmoidal curve.
Rationale: The use of a sigmoidal calibration curve depends on the calibrators that are used for the assay. This feature of immunoassays has no bearing on producing false low (or high) values.
D. Multiplex enzyme-linked immunosorbent assay (ELISA) used to perform this assay.
Rationale: Multiplex assays allow for the measurement of more than one analyte simultaneously. They do not necessarily result in interferences between the analytes.
E. Presence of the hook effect.
Rationale: The hook effect occurs when the analyte of interest is present in very high concentrations, such that antigen molecules (i.e., the analyte) are bound to both the capture and detection antibodies. In such a scenario, a proportional signal cannot be generated and the resulting signal will be falsely low.
Rationale: The beta fraction contains proteins such as transferrin and complement components C3 and C4.
B. Presence of a band in the gamma globulin fraction at a concentration of 3 g/dL.
Rationale: The gamma globulin fraction contains immunoglobulins, which are elevated as a monoclonal spike(s) in multiple myeloma.
C. Decreased level of a band in the albumin fraction to a concentration of 0.2 g/dL.
Rationale: The albumin fraction contains albumin, the most abundant protein in serum. The condition associated with a decrease in this fraction is referred to as hypoalbuminemia.
D. Increased levels of bands in the alpha-1 and alpha-2 globulin fractions at a concentration of 0.2 g/dL each.
Rationale: The alpha fractions contain proteins such as haptoglobin, which are acute-phase reactants, and which can increase in the setting of inflammation or infection.
E. Presence of a band in the prealbumin fraction at a concentration of 0.5 g/dL.
Rationale: The prealbumin fraction contains one protein (prealbumin or, equivalently, transthyretin), which is unrelated to multiple myeloma.
B. Decreased albumin, increased alpha-1 and alpha-2 globulins, and normal beta and gamma globulins.
C. Decreased albumin, decreased alpha-1 and alpha-2 globulins, and normal beta and gamma globulins.
D. Increased albumin, decreased alpha-1 and alpha-2 globulins, normal beta globulins, and increased gamma globulins.
E. Normal albumin; increased alpha-1, alpha-2, and beta globulins; and normal gamma globulins.
Rationale: In acute inflammation, albumin is usually decreased, alpha-1 and alpha-2 globulins are increased, and beta and gamma globulins are normal.
Rationale: This is the major site for AFP synthesis up to approximately 10 weeks of gestation.
B. The fetal pancreas.
Rationale: AFP is not synthesized in the fetal pancreas.
C. The yolk sac and fetal liver.
Rationale: AFP is not synthesized in the yolk sac after 12 weeks of gestation.
D. The fetal liver.
Rationale: This is the major site of AFP synthesis after 12 weeks of gestation.
E. The fetal kidney.
Rationale: AFP is not synthesized in the fetal kidney.
Rationale: In liver disease, the gamma globulin fraction is usually elevated.
B. This is the serum IFE pattern for a monoclonal protein.
Rationale: The IFE pattern shows the presence of polyclonal immunoglobulins.
C. This is the serum protein electrophoresis pattern that is usually seen in nephrosis.
Rationale: This is a serum protein electrophoresis pattern with a low albumin, elevated alpha-2 globulin, a normal or decreased gamma globulin fraction, and a low total protein concentration is usually indicative of nephrosis.
D. This is the serum protein electrophoresis pattern that is seen in iron deficiency anemia.
Rationale: In iron deficiency anemia, the total protein concentration and the proteins in the alpha-2 region are usually normal.
E. This is the serum protein electrophoresis pattern that is seen in alpha-1 antitrypsin deficiency.
Rationale: It is not alpha-1 antitrypsin deficiency, because the proteins in the alpha-1 region are actually increased in concentration.
B. Specificity.
C. Precision.
D. Negative predictive value.
E. Accuracy.
Rationale: See Major Points of Discussion.
B. Specificity.
C. Positive predictive value.
D. Negative predictive value
E. Accuracy.
Rationale: See Major Points of Discussion.
B. Precision.
C. Positive predictive value.
D. Reference interval.
E. Accuracy.
Rationale: See Major Points of Discussion.
B. Precision.
C. Positive predictive value.
D. Negative predictive value.
E. Accuracy.
Rationale: See Major Points of Discussion.
B. Specificity.
C. Positive predictive value.
D. Negative predictive value.
E. Accuracy.
Rationale: See Major Points of Discussion.
B. SPEP showed low albumin and slightly elevated gamma globulin levels and the possibility of a monoclonal protein in the gamma globulin region, which was identified by IFE as a free lambda light chain.
Rationale: A monoclonal protein is not seen by IFE.
C. SPEP showed low albumin and slightly elevated polyclonal gamma globulin levels. No monoclonal protein was detected by IFE.
Rationale: SPEP showed the possibility of a monoclonal protein in the gamma globulin region.
D. SPEP showed low albumin and slightly elevated gamma globulin levels and the possibility of a monoclonal protein in the gamma globulin region. No monoclonal protein was detected by IFE. The narrow band in the SPEP pattern is probably due to fibrinogen.
Rationale: This is the correct interpretation.
E. SPEP showed low albumin and slightly elevated gamma globulin levels and the possibility of a monoclonal protein in the gamma globulin region, which was identified by IFE as a monoclonal IgA lambda protein.
Rationale: A monoclonal IgA lambda protein is not seen by IFE.
B. Acquisition of this mutation marks the transition from early-stage to late-stage disease.
Rationale: No such marker currently exists that can accurately characterize this transition.
C. This mutation correlates with sensitivity to chemotherapy using tyrosine kinase inhibitors.
D. This mutation correlates with resistance to chemotherapy using tyrosine kinase inhibitors.
Rationale: The T790M mutation has been identified in tumors that have developed resistance to tyrosine kinase inhibitors.
E. This mutation portends a favorable outcome (i.e., is an indicator of good prognosis).
Rationale for C and E: The L858R mutation was initially found to be an indicator of a poor prognosis. It was later found that tumors harboring this mutation were sensitive to tyrosine kinase inhibitors.
B. Acquisition of this mutation marks the transition from early-stage to late-stage disease.
Rationale: No such marker currently exists that can accurately characterize this transition.
C. This mutation correlates with sensitivity to chemotherapy using tyrosine kinase inhibitors.
D. This mutation correlates with resistance to chemotherapy using tyrosine kinase inhibitors.
Rationale: The T790M mutation was identified in tumors that developed resistance to tyrosine kinase inhibitors.
E. This mutation portends a favorable outcome (i.e., is an indicator of good prognosis).
Rationale for C and E: The L858R mutation was initially found to be an indicator of a poor prognosis. It was later found that tumors harboring this mutation were sensitive to tyrosine kinase inhibitors.
Rationale: CA-15.3 is FDA approved for breast cancer monitoring.
B. PSA.
Rationale: PSA is FDA approved for prostate cancer monitoring.
C. AFP.
Rationale: AFP is used in monitoring patients with HCC and for patients with germ cell tumors.
D. hCG.
Rationale: hCG is primarily used in monitoring patients with germ cell tumors.
E. CEA.
Rationale: CEA is used to monitor disease in patients with lung cancer.
Rationale: CEA is not useful for diagnostic purposes but is an important tumor marker for monitoring patients with lung cancer.
B. CEA has poor specificity for lung cancer; there are multiple nonmalignant conditions resulting in elevated CEA levels.
Rationale: Smoking and multiple inflammatory conditions are characterized by elevated circulating levels of CEA.
C. The dynamic range of this assay is limited and not suitable for patients with late-stage or metastatic disease.
Rationale: The dynamic range of an assay should not affect screening for disease.
D. The analytical sensitivity of this assay does not meet the needs for early detection.
Rationale: Although analytical sensitivity may impact early detection, this is not related to the problems related to population-based screening.
E. The assay is imprecise (i.e., the coefficient of variation is > 20%).
Rationale: Assay (im)precision does not directly affect population-based screening.
Rationale: Maternal serum AFP levels are usually elevated in anencephaly and myelomeningocele.
B. Anencephaly, myelomeningocele, and closed spina bifida.
C. Anencephaly and closed spina bifida.
Rationale: Screening for closed spina bifida using maternal serum AFP levels is not possible because the maternal serum AFP level is not elevated.
D. Trisomy 21.
E. Trisomy 18.
Rationale: A single biomarker is not used to screen for trisomy 21 or trisomy 18.
Rationale: Alpha-1-antitrypsin is a positive acute-phase protein, the concentration of which increases in the setting of inflammation.
B. Albumin and alpha-2-macroglobulin.
Rationale: Alpha-2-macroglobulin is a protease inhibitor and is a positive acute-phase protein.
C. Haptoglobin and CRP.
Rationale: Haptoglobin and CRP are positive acute-phase proteins, the concentrations of which increase in the setting of inflammation.
D. Albumin and transferrin.
Rationale: These are both negative acute-phase proteins, the concentrations of which decrease in the setting of inflammation.
E. Transferrin and C3.
Rationale: C3, a component of the complement system, is a positive acute-phase protein, the concentration of which increases in the setting of inflammation.
Rationale: AFP is not used in the first trimester to screen for fetal Down syndrome.
B. NT, free beta-hCG, and pregnancy-associated protein A (PAPP-A).
Rationale: These are the markers that are used to screen for fetal Down syndrome in the first trimester.
C. NT, free beta-hCG, and inhibin A.
D. NT, free beta-hCG, and unconjugated estradiol (uE3).
Rationale: uE3 is not used in the first trimester to screen for fetal Down syndrome.
E. NT, PAPP-A, and inhibin A.
Rationale for C and E: Inhibin A is not used in the first trimester to screen for fetal Down syndrome.
Rationale: Vitamin A is not associated with NTDs. Vitamin A promotes vision and plays a role in humoral immunity.
B. Folic acid.
Rationale: The introduction of folic acid into foods in 1998 significantly decreased the occurrence of NTDs in the United States.
C. Vitamin D.
Rationale: Vitamin D is not associated with NTDs. Vitamin D is needed for bone growth and development.
D. Vitamin K.
Rationale: Vitamin K is not associated with NTDs. Vitamin K is important in the synthesis of proteins required for blood clotting.
E. Vitamin E.
Rationale: Vitamin E is not associated with NTDs. Vitamin E is an antioxidant that functions as a free radical scavenger.
B. Increased, because the MOM will be lower than expected.
Rationale: The MOM calculated using the median MSAFP value at 19 weeks of gestation would be lower than expected when the true gestational age is 15 weeks, because MSAFP levels increase with increasing gestational age.
C. Decreased, because the MOM will be lower than expected.
Rationale: Overestimation of gestational age would decrease both the MOM and risk of an NTD.
D. Decreased, because the MOM will be higher than expected.
Rationale: The MOM would be lower than expected, because MSAFP levels increase with increasing gestational age.
E. Cannot be determined; therefore, another sample should be drawn to repeat the MSAFP determination.
Rationale: Another sample should not be drawn. The risk for a fetal NTD can be recalculated from the original MSAFP result by using the correct gestational age.
B. Increased, because the actual MOM is lower than the calculated MOM.
C. Decreased, because the actual MOM is lower than the calculated MOM.
D. Decreased, because the MOM is higher than the calculated MOM.
Rationale: The MOM calculated using the median AFP value at 20 weeks of gestation would be higher than expected when the true gestational age is 15 weeks, because the AFAFP levels decrease with increasing gestational age. Therefore, the actual risk is lower than the calculated risk using the incorrect gestational age.
E. Slightly decreased, but will not significantly affect the NTD risk calculation.
Rationale: The NTD risk at 15 and 20 weeks of gestation is significantly different because of the change in AFP levels between 15 and 20 weeks of gestation.
B. Increased serum MSAFP level and decreased risk of NTDs.
Rationale: The serum MSAFP levels are lower in overweight women, because of an increase in maternal plasma volume.
C. Decreased serum MSAFP level and increased risk of NTDs.
Rationale: The risk of NTDs is lower when MSAFP levels are decreased.
D. Decreased serum MSAFP level and decreased risk of NTDs.
Rationale: The maternal plasma volume is higher in overweight patients, resulting in lower than expected serum MSAFP levels. Therefore, a lower than expected risk for NTDs would be obtained if the weight correction were not performed.
E. No change in the risk for NTDs.
Rationale: Any change in the MSAFP levels affects the risk for NTDs.
B. Proteins have a positive charge and migrate toward the cathode.
C. Proteins have a negative charge and migrate toward the anode.
Rationale: Proteins have a negative charge at a pH of 8.6 and migrate toward the positively charged anode.
D. Proteins have a negative charge and migrate toward the cathode.
Rationale: At a pH of 8.6, proteins migrate toward the anode.
E. Proteins all have the same isoelectric point.
Rationale: Proteins have different isoelectric points.
Rationale: AFP is low in Down syndrome.
B. Elevated inhibin A; low AFP, hCG, and uE3.
Rationale: Low AFP, hCG, and uE3 levels are seen in trisomy 18. hCG is elevated in Down syndrome.
C. Elevated AFP; low or normal uE3, hCG, and inhibin A.
Rationale: This is the pattern that is seen in with fetal neural tube defects. hCG and inhibin A are elevated and AFP is low in Down syndrome.
D. Elevated inhibin A and uE3; low AFP and hCG.
Rationale: hCG is elevated in Down syndrome. uE3 is decreased in Down syndrome.
E. Elevated hCG and inhibin A; low AFP and uE3.
Rationale: Elevated hCG and inhibin A; low AFP and uE3 are seen in Down syndrome.
Rationale: False-negative results are obtained with both screening methods.
B. There is an increase in the detection rate of fetal Down syndrome.
Rationale: More cases of Down syndrome are detected using the quadratic screen.
C. The reagent cost is significantly reduced.
Rationale: The reagent cost is higher using the quadratic screen.
D. Fetal trisomy 18 cases can be detected.
Rationale: Both the quadratic screen and triple screen are used to detect cases of fetal trisomy 18.
E. The analytical method is automated.
Rationale: The quadratic screen and triple screen methods can both be automated.
Rationale: This pattern can be seen when a fetus has an NTD.
B. AFP is very elevated; uE3 and hCG are low.
Rationale: This pattern can be seen when a fetus has anencephaly.
C. uE3 and hCG are elevated; AFP is low.
Rationale: uE3 is not elevated in trisomy 18.
D. AFP, uE3, and hCG are all low.
Rationale: This is the pattern that is seen when a fetus has trisomy 18.
E. hCG is elevated; AFP and uE3 are low.
Rationale: This is the pattern that is seen when a fetus has trisomy 21.
Rationale: AFP levels can range from the ng/mL (in the general population) to the mg/mL range (in late-stage liver cancer patients). Thus, an assay with a wide dynamic range is important.
B. Analytical sensitivity at the low end.
Rationale: Analytical sensitivity at the low limit of detection would not be sufficient in this situation; the high end of the range is also important.
C. High precision.
D. Minimal analytical interferences from drugs.
Rationale: These are desirable but would not be specifically relevant for this scenario.
E. Standardized results between different assays.
Rationale: As long as the same assay is used to measure the values in the entire population, a lack of standardization between different assays measuring the same analyte is not relevant in this situation.
B. Analytical sensitivity at the low end.
Rationale: Detection of recurrence after radical prostatectomy requires robust performance at the lower end of the analytical range.
C. High precision.
Rationale for A and C: Although this is an important parameter for all assays, it is not the most important characteristic for this situation. Detection of recurrence after radical prostatectomy requires robust performance at the lower end of the analytical range.
D. Minimal analytical interferences from drugs.
Rationale: Although this is important for any assay, it is not necessarily relevant for the current scenario.
E. Standardized results between different assays.
Rationale: As long as the same assay is used to measure the values when following any one individual patient, this attribute may not be necessary.
Rationale: A wide dynamic range would not explain these differences in results between these two different assays.
B. Analytical sensitivity at the low end.
Rationale: Analytical sensitivity at the low end would help in measuring low levels of analyte near the lower limit of detection but does not explain the different results obtained using these two different assays.
C. High precision.
Rationale: High precision would help with the reproducibility on any one platform but does not explain the differences in results between these two assays.
D. Minimal analytical interferences from drugs.
Rationale: Minimal interference with drugs is important is important for any assay but does not explain the difference in results between these two different assays.
E. Lack of standardization among different assays.
Rationale: In this case, the assays are different and, for example, use different capture and detection antibodies, which measure slightly different epitopes. This results in different values being measured for this analyte with the two different assays.
Rationale: Although a wide dynamic range may be generally important for tumor marker assays, it is not the most important characteristic for this situation.
B. Analytical sensitivity at the low end.
Rationale: This is not important in this situation, where analyte levels are being measured and compared in the elevated to higher end of the range, not the lower end of the range.
C. High precision.
Rationale: This is most critical for serial assessment of specific patients, as levels of the tumor marker can be compared before, during, and after completion of the treatment regimen.
D. Minimal analytical interferences from drugs.
Rationale: This is generally important for any assay, but not specifically for this scenario.
E. Lack of standardization among different assays.
Rationale: Standardization between different assays is not necessary for this scenario.
Rationale: This is the definition of type I cryoglobulinemia.
B. Detection of albumin and a monoclonal protein in the cryoprecipitate.
Rationale: Albumin is not included in the definition of type II cryoglobulinemia.
C. Detection of alpha-1, alpha-2, and beta globulins in the cryoprecipitate.
Rationale: These proteins are not included in the definition of type II cryoglobulinemia.
D. Detection of polyclonal immunoglobulins in the cryoprecipitate.
Rationale: This is the definition of type III cryoglobulinemia.
E. Detection of a monoclonal protein and polyclonal immunoglobulins in the cryoprecipitate.
Rationale: See Major points of discussion.
Rationale: Urinary copper concentrations are elevated in Wilson disease.
B. Low serum ceruloplasmin and elevated urinary copper concentrations.
Rationale: Low serum ceruloplasmin and elevated urinary copper concentrations are characteristic of Wilson disease.
C. Elevated serum ceruloplasmin and low urinary copper concentrations.
D. Elevated serum ceruloplasmin and elevated urinary copper concentrations.
E. Normal serum ceruloplasmin and low urinary copper concentrations.
Rationale: Serum ceruloplasmin concentrations are low and urinary copper concentrations are elevated in Wilson disease.
Rationale: CA-125 is useful for monitoring ovarian cancer, not breast cancer.
B. BRCA1/2.
C. BRCA1 only.
Rationale: Genotyping for BRCA1 and BRCA2 is useful to assess predisposition to breast and ovarian cancers.
D. CA-15.3.
Rationale: CA-15.3 is the best tumor marker for monitoring breast cancer patients.
E. EGFR genotyping.
Rationale: EGFR genotyping is useful to predict response to therapy in patients with NSCLC.
Rationale: CA-125 is the best tumor marker for monitoring ovarian cancer.
B. HE4.
Rationale: HE4 is a new biomarker for monitoring ovarian cancer but is not yet the standard of care for this disease.
C. BRCA1/2.
Rationale: Genotyping for BRCA1 and BRCA2 is useful to assess predisposition of breast and ovarian cancers.
D. CA-15.3.
Rationale: CA-15.3 is the best tumor marker for monitoring breast cancer patients but is not useful for evaluating patients with ovarian cancer.
E. EGFR genotyping.
Rationale: EGFR genotyping is useful to assess patients with NSCLC.
Rationale: PSA combined with DRE is approved for screening purposes but may not necessarily be useful for monitoring.
B. PSA density combined with PC3.
Rationale: PSA density is obtained through imaging analysis and serum PSA measurement. PC3 is a new molecular-based prostate cancer biomarker measured in urine.
C. PSA combined with PC3.
Rationale: PC3, a new molecular-based prostate cancer biomarker measured in urine, has not yet been demonstrated to be useful in this setting.
D. PSA combined with circulating tumor cells (CTCs).
Rationale: Both markers are approved by the FDA for monitoring purposes.
E. No two modalities together; PSA alone is best.
Rationale: Although PSA alone is satisfactory, combining this test with CTCs is the best approach.
Rationale: Hypogammaglobulinemia can be seen in patients who have a monoclonal light chain clone that suppresses the production of normal polyclonal immunoglobulins. The abnormal monoclonal light chain is usually not seen in plasma but can be detected in urine.
B. Urine protein electrophoresis should not be performed using an aliquot from a random urine collection.
Rationale: Urine protein electrophoresis can use either a random or a 24-hour collection to identify the presence of a monoclonal protein. However, a 24-hour urine collection is necessary to determine the total amount of protein secreted. Therefore, in the latter case, it is necessary to know the total amount of urine produced during a 24-hour period.
C. Bence-Jones proteinuria is a diagnostic marker for multiple myeloma.
Rationale: The detection of monoclonal light chains in the urine (i.e., Bence-Jones proteinuria) has been used as a diagnostic marker for multiple myeloma since the report by Dr. Henry Bence-Jones in 1847.
D. The monoclonal light chains are found in concentrated urine in very small amounts and cannot be quantified as an M-spike by protein electrophoresis.
Rationale: Serum free light chain determination is based on antibody binding to epitopes that are normally hidden by association with heavy chains. Light chains are normally synthesized in slight excess compared with heavy chains but are rapidly cleared from the circulation so that they do not accumulate in serum/plasma.
E. Determination of serum free light chains is sufficient to diagnose Bence-Jones proteinuria.
Rationale: Bence-Jones proteinuria is defined as the presence of monoclonal light chains in the urine.
B. No abnormalities in lane 1; IgG kappa in lane 2; IgA lambda in lane 3.
C. Bisalbuminemia in lane 1; abnormal band in the gamma region in lanes 2 and 3.
Rationale for B and C: A “split” albumin band is seen in lane 1. Although lanes 2 and 3 each show a monoclonal spike in the gamma region, it is not possible to determine the constituent heavy and light chains by standard serum protein electrophoresis; IFE is required for this purpose.
D. Hypogammaglobulinemia in lane 1; hypergammaglobulinemia in lanes 2 and 3.
Rationale for A and D: Gamma globulins migrate in the gamma region of the gel, which is most cathodal; the concentration of these proteins is not elevated in lane 1. Lanes 2 and 3 each show a monoclonal spike in the gamma region. A split albumin band is seen in lane 1.
E. No abnormalities in lane 1; IgA lambda in lane 2; IgG kappa in lane 3.
Rationale: A split albumin band is seen in lane 1. Although lanes 2 and 3 each show a monoclonal spike in the gamma region, it is not possible to determine the constituent heavy and light chains by standard serum protein electrophoresis; IFE is required for this purpose.
Rationale: Cryoglobulinemia is not associated with normal childbirth.
B. A 45-year-old patient with diabetes mellitus.
Rationale: Cryoglobulinemia is not associated with diabetes.
C. A 45-year-old patient with hepatitis A.
Rationale: Cryoglobulinemia is not typically associated with hepatitis A.
D. A 45-year-old patient with hepatitis C.
Rationale: Hepatitis C is commonly associated with type II cryoglobulinemia and is seen in approximately 35% of such cases.
E. A 5-year-old child with paroxysmal cold hemoglobinuria.
Rationale: Cryoglobulinemia is not associated with paroxysmal cold hemoglobinuria. In this setting, the pathologic antibody binds to red blood cells at cold temperatures but does not precipitate in the cold.
 Polyarteritis nodosa
Polyarteritis nodosa
 Rheumatoid arthritis
Rheumatoid arthritis
 Kidney disease
Kidney disease
 Leukemia
Leukemia
B. Transport at 37°C and incubate at 4°C.
C. Transport at room temperature and incubate at 4°C.
D. Transport at 4°C and incubate at 37°C.
E. Transport at 4°C and incubate at 4°C.
Rationale: To obtain accurate results regarding the presence or absence of a cryoprecipitate, the patient’s blood sample must be maintained at 37°C before reaching the performing laboratory. Once in the laboratory, the sample is centrifuged at 37°C, and then the resulting serum is incubated at 4°C.
Rationale: Albumin is the most abundant, and most anodal, fraction on the gel.
B. Beta fraction.
C. Gamma fraction.
Rationale: This fraction is most cathodal. Most immunoglobulins migrate in the gamma fraction. There are almost no other proteins that migrate in this fraction.
D. Alpha-1 fraction.
E. Alpha-2 fraction.
Rationale for B, D, and E: Most immunoglobulins migrate in the gamma fraction.
Rationale: This finding can be seen in a patient with a monoclonal light chain disorder. A monoclonal light chain disorder can result from the replacement of normal plasma cells, which produce polyclonal immunoglobulins, by a malignant clone(s). Monoclonal free light chains are cleared rapidly and SPEP is typically not sensitive enough to detect these. Therefore, urine should be evaluated for the presence of monoclonal free light chains (i.e., Bence-Jones proteins).
B. Lane 14 demonstrates the presence of increased amounts of polyclonal immunoglobulins.
Rationale: This lane shows the presence of a sharp band consistent with a monoclonal spike, not the presence of increased amounts of polyclonal immunoglobulins. Polyclonal increases in immunoglobulins have been associated with (Job syndrome), Wiskott-Aldrich syndrome, and AIDS.
C. Lane 12 demonstrates a band in the gamma region that represents fibrinogen.
Rationale: Although there should be no fibrinogen remaining in serum, remnants of fibrinogen due to incomplete clotting can result in the false-positive interpretation of a monoclonal band. The band in this lane represents a low concentration of a monoclonal spike.
D. Lane 18 demonstrates the presence of polyclonal hypergammaglobulinemia.
Rationale: The protein concentration in the gamma globulin region is actually decreased. Nonetheless, polyclonal increases in immunoglobulins have been associated with many diseases. These include immunodeficiency diseases such as hyperimmunoglobulin E (Job syndrome), Wiskott-Aldrich syndrome, and AIDS.
E. Lane 20 demonstrates hypoalbuminemia.
Rationale: Albumin is the fastest band migrating toward the anode (at the bottom of the figure); the albumin level appears to be normal in this case.
Rationale: The hyperviscosity syndrome can be seen in the setting of multiple myeloma or Waldenström macroglobulinemia.
B. The circulating concentration can be so high that the precipitating complexes by IFE will not stain properly.
Rationale: The circulating concentration can be so high that there is not enough reagent antibody to form precipitating complexes on IFE. This is analogous to the “hook effect” in clinical chemistry or the “pro-zone effect” in immunohematology.
C. Quantitative monitoring clearly differentiates a benign from a malignant condition in the majority of cases.
Rationale: This approach is important in differentiating multiple myeloma from MGUS. However, it is helpful, but not diagnostic.
D. They are routinely detected using mass spectrometry methods.
Rationale: Clinical laboratories do not routinely use mass spectrometry approaches to measure large molecules, such as antibodies.
E. Identification of the constituent heavy chain and light chain types can be determined by IFE.
Rationale: To identify the type of immunoglobulin, monospecific antisera are applied directly to the separated serum proteins. The gel is washed, and the protein-antibody conjugates are stained and read directly.
B. 1. Gamma fraction; 2. alpha-1; 3. alpha-2; 4. beta-1; 5. beta-2; 6. albumin.
C. 1. Albumin; 2. alpha-1; 3. alpha-2; 4. beta-1; 5. beta-2; 6. gamma fraction.
D. 1. Alpha-1; 2. alpha-2; 3. beta-1; 4. beta-2; 5. gamma fraction; 6.albumin.
E. 1. Gamma fraction; 2. alpha-1; 3. alpha-2; 4. beta-1; 5. albumin; 6. beta-2.
Rationale: The correct electrophoretic migration order from anode to cathode is: albumin, followed by alpha-1, followed by alpha-2, followed by beta-1, followed by beta-2, followed by the gamma fraction.
B. RF consists of antigens that are bound to the Fc portion of IgG.
Rationale: RFs are autoantibodies that bind to the Fc portion of IgG.
C. RF can cause a false-positive result with some immunochemical assays that measure hormones and drugs.
Rationale: See Major points of discussion.
D. The diagnostic specificity of RF for the detection of rheumatoid arthritis is greater than anti-cyclic citrullinated peptide (anti-CCP).
Rationale: Anti-CCP is a more specific biomarker than RF for the detection of rheumatoid arthritis.
E. Elevated serum RF levels are seen in most patients with rheumatoid arthritis.
Rationale: RF levels can be in the reference range in the early stages of rheumatoid arthritis.
Rationale: Patients with myositis rarely exhibit a centromeric staining pattern.
B. Systemic sclerosis/CREST syndrome (calcinosis, Raynaud phenomenon, esophageal dysmotility, sclerodactyly, and telangiectasia).
Rationale: Anti-centromeric antibodies are common (44% to 98%) in patients with scleroderma, specifically CREST.
C. Systemic lupus erythematosus (SLE).
Rationale: SLE patients exhibit positivity to double-stranded DNA (dsDNA), which reveals a homogeneous staining pattern.
D. Drug-induced (procainamide and/or hydralazine) lupus-like syndrome.
Rationale: This condition usually results in a homogeneous pattern.
E. Chronic active hepatitis.
Rationale: Patients with hepatitis rarely exhibit a centromeric staining pattern.
Rationale: Sjögren syndrome patients usually exhibit a nucleolar staining pattern.
B. Systemic sclerosis/CREST syndrome (calcinosis, Raynaud phenomenon, esophageal dysmotility, sclerodactyly, and telangiectasia).
Rationale: Patients with CREST usually exhibit a centromeric staining pattern.
C. SLE.
Rationale: Patients with SLE can exhibit a homogeneous (or peripheral) staining pattern.
D. Asthma.
Rationale: Asthma is not necessarily characterized by homogeneous staining patterns.
E. Chronic active hepatitis.
Rationale: Chronic active hepatitis is not necessarily characterized by homogeneous staining patterns.
Rationale: Patients with myositis do not commonly exhibit a nucleolar pattern.
B. Systemic sclerosis/CREST syndrome (calcinosis, Raynaud phenomenon, esophageal dysmotility, sclerodactyly, and telangiectasia).
Rationale: These patients usually exhibit a centromeric staining pattern.
C. SLE.
Rationale: Patients with SLE will usually exhibit a peripheral or homogeneous staining pattern.
D. Drug-induced (procainamide and/or hydralazine) lupus-like syndrome.
Rationale: This will usually exhibit a homogeneous staining pattern.
E. Sjögren syndrome.
Rationale: A majority of patients with Sjögren syndrome usually exhibit a nucleolar pattern.
Rationale: Patients with Sjögren syndrome commonly exhibit a nucleolar pattern.
B. Systemic sclerosis/CREST syndrome (calcinosis, Raynaud phenomenon, esophageal dysmotility, sclerodactyly, and telangiectasia).
Rationale: This usually exhibits a centromeric staining pattern.
C. SLE.
Rationale: This usually exhibits a peripheral or homogeneous staining pattern.
D. Drug-induced (procainamide and/or hydralazine) lupus-like syndrome.
Rationale: This usually exhibits a homogeneous staining pattern.
E. Many antigens and connective tissue diseases.
Rationale: Of all the IF patterns observed in ANA testing, the speckled pattern is the most nonspecific and is observed in multiple conditions.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree





